Desmosomes in Cancer
Desmoglein 1 in Keratinocyte-Melanocyte Cross Talk and Role in Melanoma
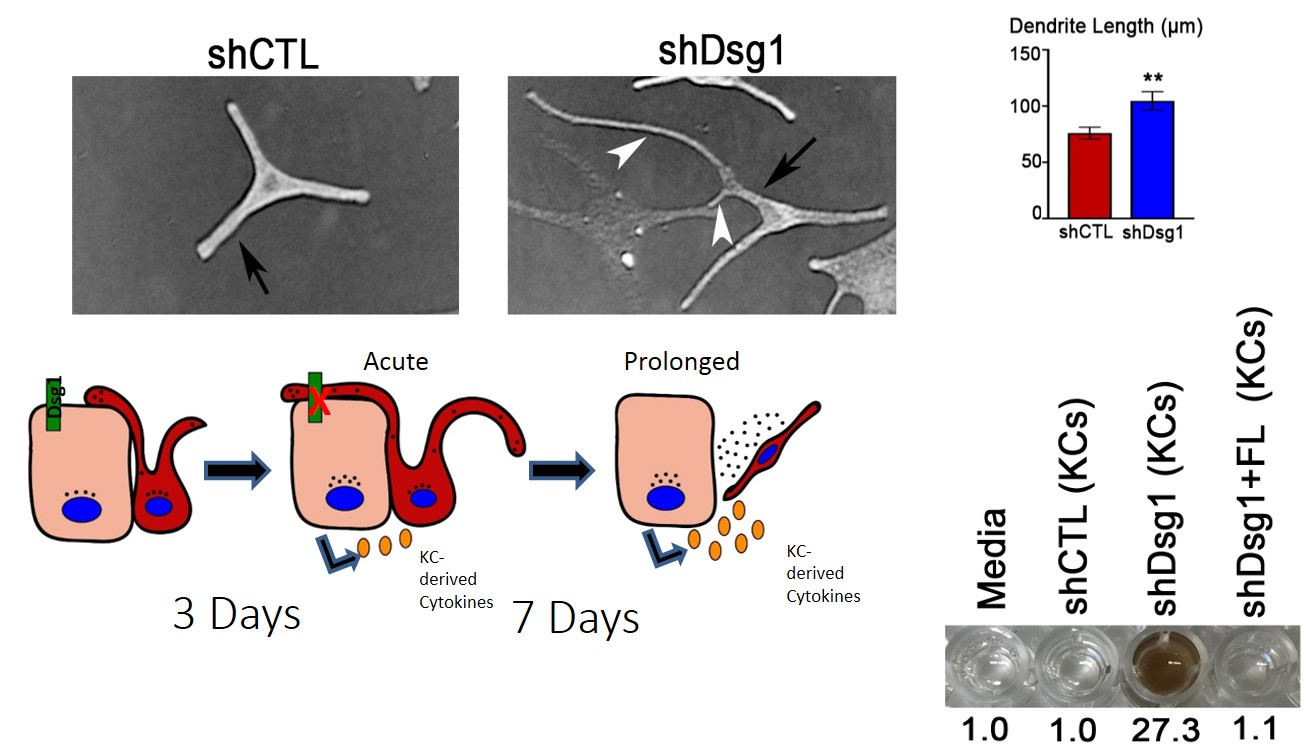
Fig. 1. MCs exposed to conditioned media from Dsg1-deficient KCs change morphology and behavior. MCs were cultured overnight in media from shCtl or shDsg1-infected KCs differentiated for 3-5 days in 1.2mM calcium medium and mixed 1:1 with MC growth media. MCs cultured with Dsg1 KD media exhibited longer dendrites compared to shCtl after 12 hrs, but shorter dendrites (a hallmark of transformation) after 7 days. On lower right, After 7 days of incubation in media conditioned by KCs transduced with shCTL, shDsg1 or shDsg1 + FL (Full length Dsgs1) retroviruses, MC media was collected, and absorbance at 405nm measured. MCs cultured in media conditioned by Dsg1-deficient KCs secrete more pigment. Raw values were normalized to media only.
**p<0.002, ****p<0.0001.
The ability of Dsg1 to regulate keratinocyte cytokine expression prompted us to explore its role in paracrine signaling in the epidermis. We characterized cytokine expression and secreted factors in Dsg1-deficient keratinocytes and noted overlap with those produced by keratinocytes in response to ultraviolet (UV) irradiation. In addition, Dsg1, but not other cadherins, was reduced in UV-irradiated keratinocytes and organotypic cultures. Thus, we hypothesized that UV-dependent loss of Dsg1 contributes to alterations in keratinocyte cytokine expression that mediate the tanning response.
Indeed, conditioned media from Dsg1-deficient keratinocyte elicited alterations in melanocytes that resemble the tanning response including increased dendricity, elevated pigment secretion, and expression of a transcription factor important for pigment production and melanoma cancer (Fig 1). In the context of 3D organotypic cultures, normal melanocytes surrounded by Dsg1-deficient keratinocytes also exhibited changes in dendricity, pigmentation and altered their localization superficially and increased cell number, akin to transformed cells (Fig 2 below). Interestingly Dsg1 was dramatically reduced in early melanoma lesions. This reduction was first observed in atypical pigmented lesions prior to a melanoma diagnosis, raising the possibility that loss of Dsg1 in nest surrounding pigmented lesions may contribute to melanoma development through elevated paracrine signaling (Fig 3 below).
Future Objectives: We are examining the mechanisms by which Dsg1 regulates keratinocyte: melanocyte cross talk both in vitro, in an animal model resembling human melanoma, and in human tissues. Human patient specimens are being interrogated though laser capture microdissection and transcriptomic analysis of adjacent keratinocyte and melanocyte/melanoma cell to map the evolution of alterations associated with Dsg1 loss. We are collaborating with experts in keratinocyte-melanocyte interactions and melanoma on these projects including Pedram Gerami and Caroline LePoole from Northwestern as well as outside collaborators Zalfa Abdel-Malek and Sancy Leachman.
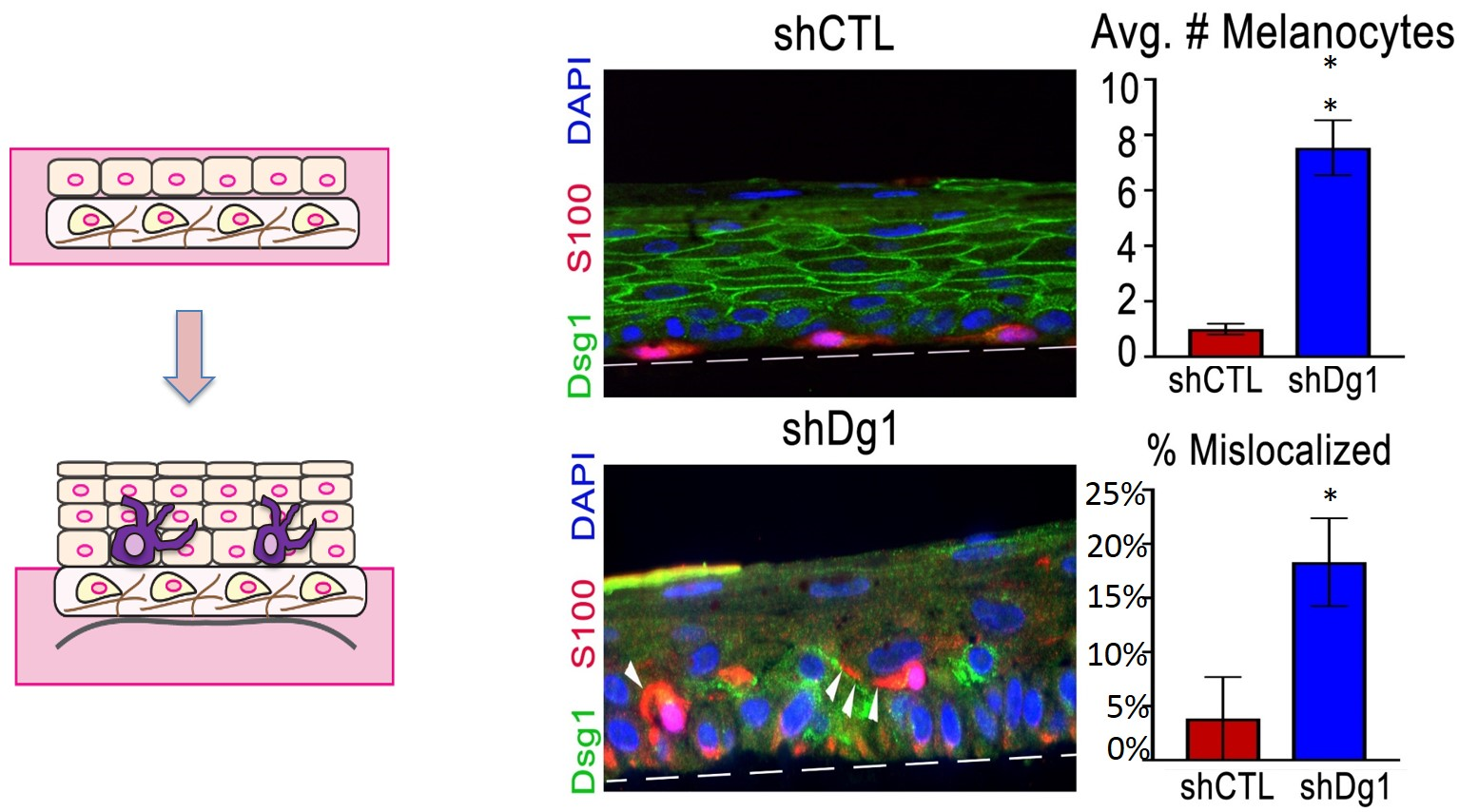
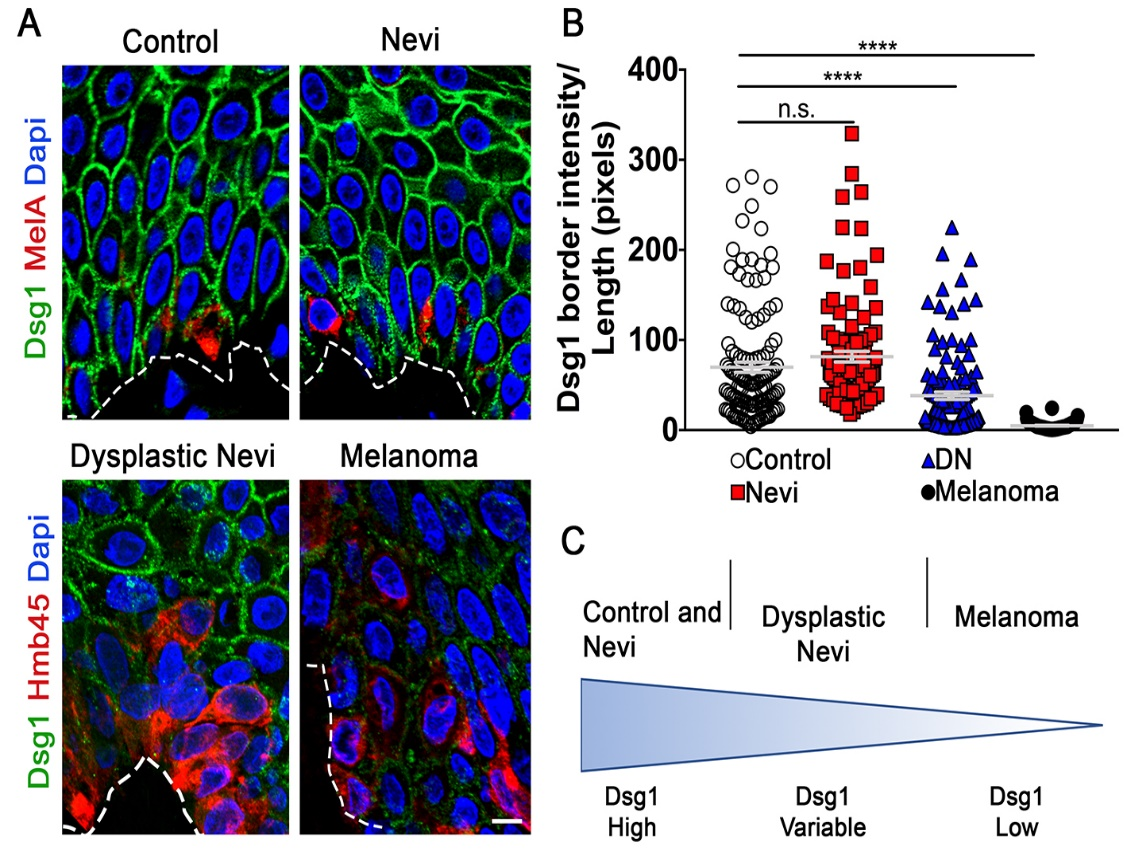
Fig. 3. KC Dsg1 is reduced in dysplastic nevi. A) Control, nevi, and matched dysplastic nevi and melanoma samples were stained for Dsg1 and the MC or melanoma markers MelA or HMB45. Scale bar 10mm. B) Mean pixel intensity for N=10 borders/condition in 4 cell rows adjacent to lesion was divided by border length in 12 samples/condition. ****p<0.0001; Kruskal-Wallis, Dunn’s post hoc test. C) Model of Dsg1 levels as a function of disease progression.