Darier's Disease
We welcome donations from people who would like to support our efforts to find a cure for Darier Disease!
Scan this QR code to donate to Darier’s Disease Research:

Support rare disease awareness through Decoding Darier's --> https://decodingdariers.com/
Instagram: @decodingdariers
LinkedIn: Decoding Dariers Disease
Facebook: Decoding Dariers
Translational Research Targeted Toward Ameliorating Consequences of SERCA2 Loss of Function in Darier's Disease
This overview describes steps directed towards normalizing the structural and immune aspects of skin caused by impaired SERCA2 function in Darier’s disease (DD). The outermost layer of the skin, the epidermis, is the first line of defense against the environment. It creates an essential barrier--guarding against water loss, sunlight, pathogens and mechanical insults. Critical for this barrier function are mechanisms that regulate the balance of extra- and intracellular calcium. The importance of calcium in maintaining epidermal balance is underscored by Darier’s disease. This human skin disorder is caused by mutations in the gene that codes for the “sarcoplasmic endoplasmic reticulum calcium” pump, SERCA2, resulting in insufficient SERCA protein. SERCA protein is important for maintaining calcium homeostasis in cells. Typically, one gene is affected, resulting in half the amount of SERCA2, known as “haploinsufficiency”.
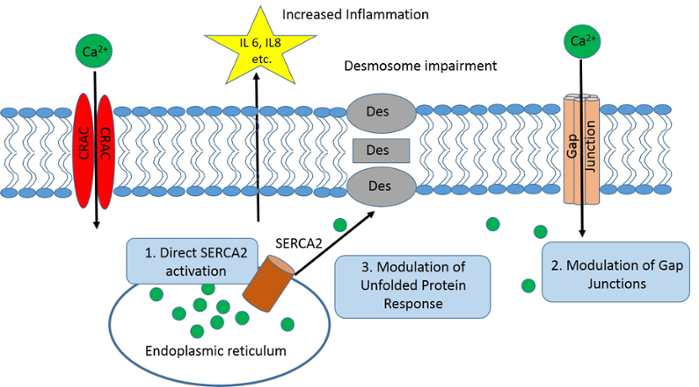
The insufficiency in SERCA2 resulting in altered calcium homeostasis has several major impacts:
- Activation of stress pathways in the cell that can impair protein structure and function;
- Loss of adhesion between cells of the epidermis due to improper assembly and regulation of the major epidermal adhesive junction—the desmosome; and
- Alterations in the immune system that lead to inflammation. The presence of inflammatory cells and increased cytokine release by keratinocytes downstream of UV irradiation can lead to further loss of the remaining SERCA2. The combined impact of weakened cell-cell adhesion and inflammation lead to the characteristic appearance associated with the disease.
Treatment options for patients with Darier’s disease are limited, and an improved understanding of the molecular basis behind the adhesive dysfunction and abnormalities in calcium signaling is necessary for the development of new therapeutic targets to ameliorate this disorder. Going forward, we will consider several alternative strategies, none mutually exclusive, which could help ameliorate the impact of SERCA2 loss on skin.
Experimental Strategies
- Target SERCA2 directly: The fact that there is a functional gene remaining in one chromosome in GL DD provides an opportunity to directly target SERCA2 to increase its activity or expression. We will use a new SERCA2 activator called CDN1163 that has been used effectively in animal models of diabetes, Parkinson’s disease and stress-related muscle wasting, to address whether the drug reverses loss of cell-cell adhesion and cytokine alterations in GL’s keratinocytes. While this drug is not yet in clinical use in humans, its broad success in multiple animal models signals movement toward further development for human treatments.
- Target intercellular channels called gap junctions: Gap junctions are channels that connect cells directly to allow passage of small molecules, including calcium. Dysregulation of gap junctions has been associated with multiple disease entities, including skin-related disorders. We will target dysfunctional gap junctions with aCT1, a small peptide mimicking a small part of a gap junction building block called connexin 43. aCT1 is used clinically for improved wound healing, and can reduce scar area, inflammatory cell infiltration, and improve the mechanical strength of the skin after wounding. In other studies, human monoclonal antibodies are being used to block hyperactive Cx26 half channels (hemichannels), which we know are elevated under conditions where desmosomes are impaired.
- Target the unfolded protein response: The unfolded protein response is a process that is stimulated when cells are under stress due to alterations in normal calcium regulation. It can lead to abnormal behavior of proteins, including aberrant trafficking within the cell. We will use Eliglustat (Cerdelga) and Miglustat (Zavesca), which can act both on calcium homeostasis and as pharmacological chaperones that improve trafficking of proteins to the cell surface. These agents are already used to treat patients with Gaucher disease and Niemann-Pick disease type C, and Miglustat has been shown to improve desmosome formation and function in Darier’s disease cells in preliminary culture studies.