Research
Corticospinal motor neurons (CSMN), also known as upper motor neurons (UMNs), have a unique ability to receive, integrate, translate, and transmit the cerebral cortex's input toward spinal cord targets and therefore act as a “spokesperson” for the initiation and modulation of voluntary movements that require cortical input. CSMN degeneration has an immense impact on motor neuron circuitry and is one of the underlying causes of numerous neurodegenerative diseases, such as frontotemporal dementia (FTD), primary lateral sclerosis (PLS), hereditary spastic paraplegia (HSP), and amyotrophic lateral sclerosis (ALS). In addition, CSMN death results in long-term paralysis in spinal cord injury patients.
Therefore, UMNs are clinically relevant and important neuron populations that require detailed investigations and better understanding. The Ozdinler Lab was established as one of the first UMNs in the nation, which focuses on understanding the biology and the pathology of this important neuron population.
Investigating NU-9, its efficacy on other diseases
Currently, there are no effective long-term treatment strategies for neurodegenerative diseases. There is an urgent need to identify compounds that would help overcome the problems vulnerable and diseased neurons are faced with. Protein aggregation is one common problem shared by many neurodegenerative diseases. Even though different proteins aggregate in different neurons, the protein aggregation problem is common. Likewise, mitochondrial problems are observed in neurons that undergo degeneration in different diseases.
We recently found that NU-9, a novel compound generated and characterized in the Silverman lab here at Northwestern, improves the health of UMNs that degenerate due to misfolded SOD1 toxicity and TDP-43 pathology, two different cellular problems. NU-9 was able to promote neuronal survival by reducing protein accumulation, problems with mitochondrial stability, endoplasmic reticulum (ER) integrity, and cytoarchitectural dynamic defects.
Since these cellular problems are also shared in other diseased neurons, it is of great interest to investigate whether NU-9 would have an impact on improving the health of other diseased neurons in other diseases. We focus our attention to ALS/FTD, FTD, and Alzheimer’s Disease (AD). In addition, we investigate other UMN diseases, such as HSP and PLS.
Investigating NU-9, its mode of action
NU-9 stands out for its ability to overcome cellular problems that occur due to misfolded SOD1 and TDP-43 pathology, in the UMNs. Most recently, Dr. Klein’s group reported NU-9’s ability to reduce amyloid beta oligomer (AβO) accumulation within the context of AD. To our knowledge NU-9 is the only compound that can act upon 3 different proteins that accumulate in 3 different diseases.
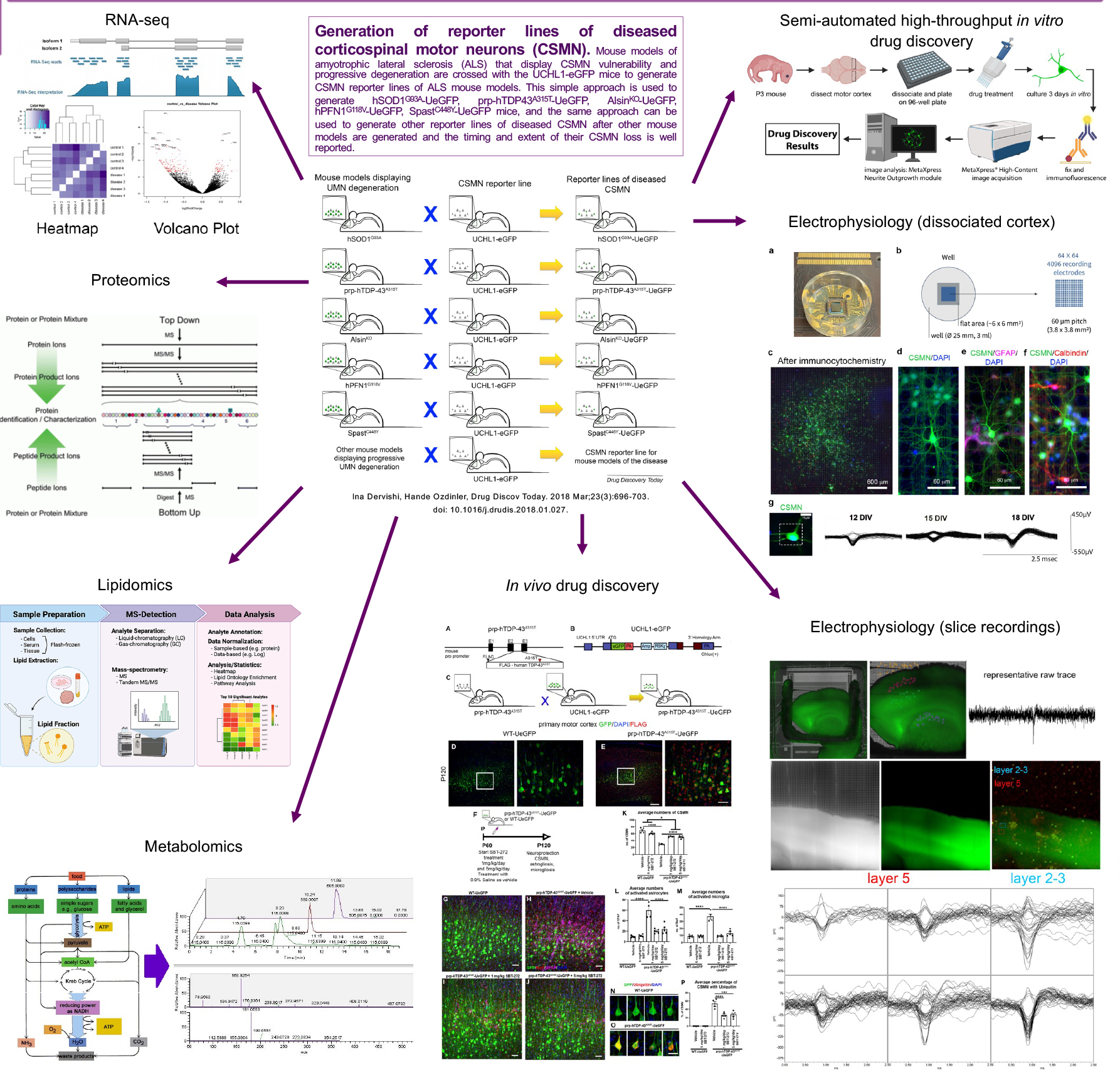
Drug discovery efforts require us to reveal NU-9’s mode of action. It is important to understand how NU-9 does what it does and how it can have such broad and significant impact on neuronal health. We utilize proteomic, metabolomic and lipidomic approaches to begin to reveal the changes that occur in the motor cortex and more selectively in the diseased UMNs with respect to treatment and how that translates to changes in the protein levels as detected in the serum and/or plasma. Such studies will not only reveal the mode of action of NU-9, but it has the potential to reveal novel pharmacokinetic biomarkers that inform about the timing and the extent of UMN loss and repair.
Developing a novel high throughput drug discovery platform using diseased UMNs
To improve the success rates of clinical trials we need to develop cell-based and mechanism-focused drug discovery efforts and investigate the survival requirement of neurons that are affected in diseases. We need to develop ways to identify compounds that improve cellular defects that occur due to a given mechanism. This requires a “mechanism focused” drug discovery effort. To improve the health of the patients, it is important to improve the health of the neurons that are diseased. This is considered a “cell-focused approach” to drug discovery.
UMNs isolated form UCHL1-eGFP reporter mice retain neuronal identity in culture. They maintain their pyramidal shape, extend a long axon and, most importantly, continue to express molecular markers, such as CTIP2. It is also very important that they diseased UMNs display diseased phenotype also in culture. Culturing dissociated cells and performing regular assays can bring effective solutions to the current challenges in ALS drug discovery. But imaging, tracing, and quantification of each neuron manually one-by-one is very time and labor intensive. Establishing the semi high-throughput drug discovery/verification platform helps us identify compounds which improve the health of UMNs that are diseased due to different underlying factors, and also to identify compounds that reduce astrogliosis and microgliosis in the motor cortex of ALS. This semi high throughput screening system allows for a more robust, unbiased and effective for cell-based and mechanism-focused drug discovery/verification platform design.
Investigating electrophysiological properties and functional connectivity of UMNs with respect to health and disease
Modulation of UMN function involves a delicate and continuous balance between excitatory and inhibitory inputs. Cortical hyperexcitation followed by in-excitability suggests the early involvement of cortical dysfunction in ALS pathology. However, a high-spatiotemporal resolution on our understanding of their functional health and connectivity is lacking. We utilize a novel high-density microelectrode array (HD-MEA) system, which incorporates dissociated cultures derived from the motor cortex or brain slices from adult mice of UCHL1-eGFP mice, in which corticospinal motor neurons (CSMN; a.k.a. UMNs in mice) are genetically labeled with eGFP expression.
We combine optical imaging with high-density microelectrode array (HD-MEA) system enabling single cell resolution and utilize UCHL1-eGFP mice to bring cell-type specificity to our understanding of the electrophysiological features of healthy UMN, as they mature and form network connections with other cortical neurons, in vitro. The HD-MEA system comprises 4096 distinct electrodes spaced 60 μm apart, enabling simultaneous recording. In addition, the HD-MEA system enables the investigation of systems as networks, utilizing graph theory, such that structural components and the functional interactions among neurons and non-neuronal cells can be quantitatively analyzed, and the roles of individual components within a network and their contributions to the overall network dynamics can be assessed. These advancements represent significant achievements in our efforts to bring cellular resolution to complex and heterogeneous structures, such as the brain. This novel approach lays the foundation for future cell-type specific analyses of UMN that are diseased due to different underlying causes with cellular precision, and it will allow the assessment of their functional response to compound treatment, especially for drug discovery efforts in upper motor neuron diseases.
Investigation of novel biomarkers for UMN diseases
We need to identify biomarkers that would inform us about the timing and the extent of upper motor neuron loss in patients, so that we can develop proper clinical trials with better outcome measures. For this study, we perform proteomic approaches to reveal the extent of proteins that are present in the serum and plasma of ALS patients with prominent UMN loss and ALS patients with prominent LMN loss, in addition to primary lateral sclerosis (PLS) patients and healthy controls. In addition, we perform proteomic analyses using pure populations of UMNs isolated at different stages of from well-defined mouse models that mimic different aspects of human pathology. This study reveals the proteins that display and increasing or decreasing profile with respect to disease, suggesting their potential association with the disease. If these proteins are also present in the blood and are conserved in the patient, they would be very valuable in the identification of potential prognostic and pharmacokinetic biomarkers that are related to UMN loss or degeneration with respect to disease progression.
Directed gene delivery to UMNs
We are developing AAV-mediated gene delivery approaches to selectively modulate the UMNs, without affecting other cells or neurons in the circuitry. Especially for ultra-rare disease patients with known mutations, gene therapy approaches are beginning to offer a potential treatment strategy. Therefore, developing gene delivery approaches are very timely.
Our lab focuses on optimizing the promoter of choice and the best mode of administration to obtain the most significant outcomes without toxicity. Our overall goal is to help develop personalized medicine approaches for HSP or PLS patients with known mutations, such that the diseased UMNs will be supported with the correct genetic intervention.