Research
Our research focuses on infection by Human Immunodeficiency Virus type 1 (HIV-1), a retrovirus and causative agent of acquired immunodeficiency syndrome (AIDS). In addition to suppressing the immune system, rendering victims susceptible to opportunistic infections, HIV-1 can cross the blood-brain barrier and cause serious damage to the central nervous system, ultimately leading to HIV-associated dementia.
Using a variety of live cell imaging approaches, we study how HIV-1 particles move within infected cells, including brain cell types such as microglia. Our goal is to understand the molecular basis behind how MTs, regulators of MT dynamics and MT motor proteins function to enable HIV-1 movement to and from the nucleus. In addition, we also study how HIV-1 infection of brain-resident cells results in the production of cytotoxic factors that contribute to neurodegeneration.
Microtubule Regulators & Their Role in Virus Infection
Our work focuses on how HIV-1 exploits MTs and MT regulatory factors to infect various natural target cells types such as microglia and primary macrophages. In particular, we are interested in how HIV-1 exploits specialized plus end-binding proteins (+TIPs) to alter MT behavior and regulate infection. To date, we have shown that HIV-1 targets a number of distinct +TIPs to coordinate the induction of MT stabilization and transport of incoming viral cores towards the nucleus with the disassembly of capsid (a poorly understood process also known as uncoating). We aim to dissect the underlying mechanisms by which these processes are coupled by distinct +TIPs during early HIV-1 infection.
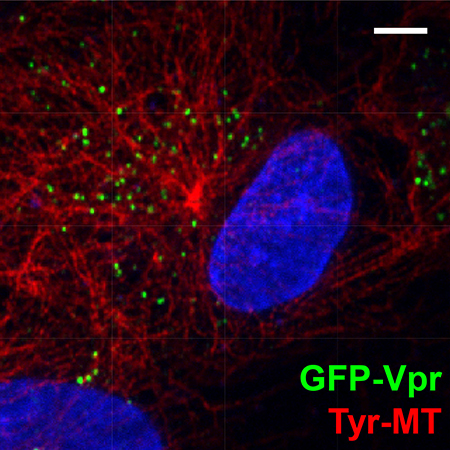
Primary human cells infected with fluorescently tagged HIV (green). Host cell nucleus is blue, microtubules are red.
The Role of the Kinesin-1 Adaptor Protein FEZ1 in HIV-1 Infection
Although it has long been known that HIV-1 uses MTs to move within infected cells, viral capsids do not appear to interact with motor proteins directly. Our laboratory initially discovered that HIV-1 capsids bind the kinesin-1 adaptor protein, FEZ1. FEZ1 bridges kinesin to particles and enables them to regulate kinesin activity. HIV-1 also recruits the kinase, MARK2 to locally regulate FEZ1 activity on the capsid. This controls both retrograde transport and the poorly understood process of HIV-1 capsid uncoating. Our current efforts are focused on understanding the mechanistic details behind how FEZ1 functions to coordinate early steps of HIV-1 infection.
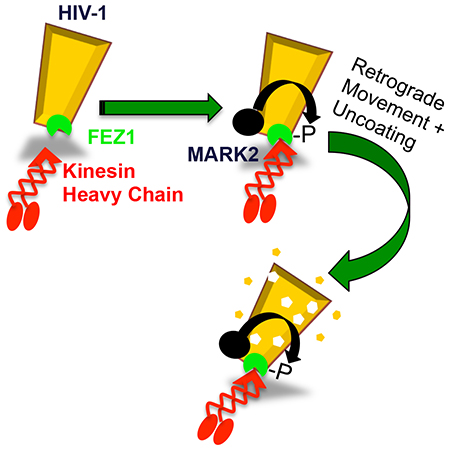
Our working model for how FEZ1 phosphorylation is regulated on the HIV-1 capsid by the kinase, MARK2.
Understanding HIV-1 Infection in the Brain & HIV-Induced Neurodegeneration
We are interested in understanding the underlying causes of HIV-associated dementia (HAD) or HIV-associated neurocognitive disorder (HAND). We recently discovered that Amyloid Precursor Protein (APP) acts as an innate restriction to HIV-1 replication in brain-resident microglia. To evade this restriction, HIV-1 exploits host secretases to enhance APP processing but in doing so results in increased production of neurotoxic beta-amyloid (Ab). Having established a novel in vitro neurodegeneration system we aim to determine the mechanisms by which HIV-1 overcomes APP-mediated restriction in microglia, and how this contributes to neurodegeneration.
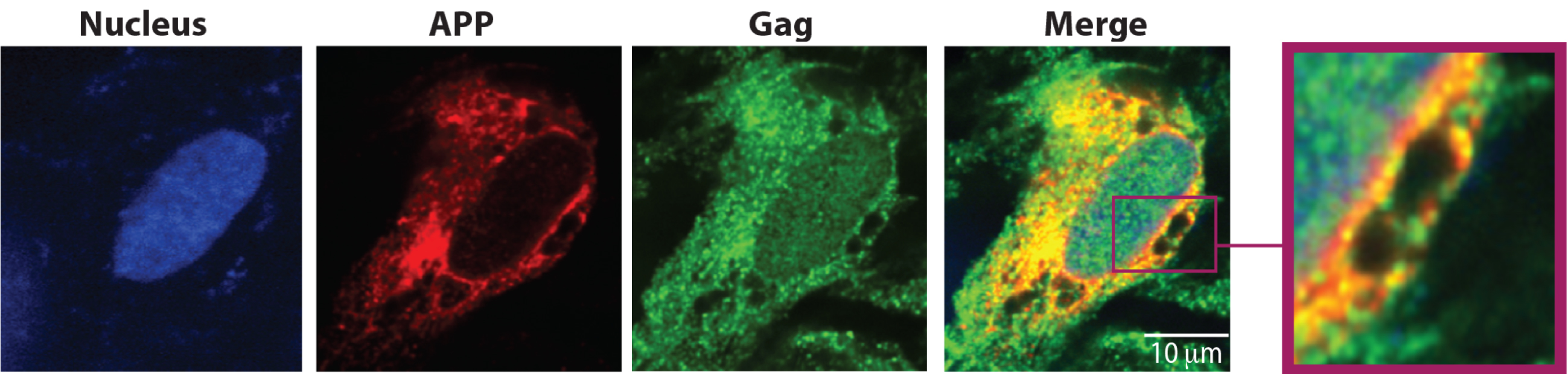
Co-localization of HIV-1 Gag and APP in microglia cells.