Research
Project 1: Genetic predisposition of primary ciliary dyskinesia
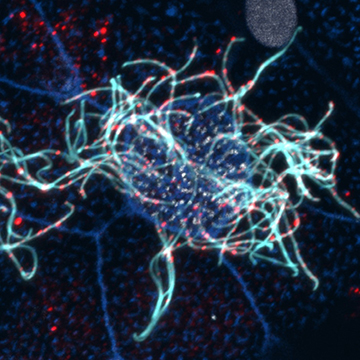 Primary Ciliary Dyskinesia (PCD) is a rare genetic disorder that affects multiple tissues leading to increased risk for situs inversus, infertility, hydrocephalus and respiratory disease. In the lung, PCD phenotypes are driven by the failure of multi-ciliated cells (MCCs) to generate proper mucociliary flow leading to chronic respiratory infections.
Primary Ciliary Dyskinesia (PCD) is a rare genetic disorder that affects multiple tissues leading to increased risk for situs inversus, infertility, hydrocephalus and respiratory disease. In the lung, PCD phenotypes are driven by the failure of multi-ciliated cells (MCCs) to generate proper mucociliary flow leading to chronic respiratory infections.
To date the combined efforts of many groups have identified over 50 genes causative for PCD. However, the diagnostic tools for identifying PCD patients are time consuming, costly, and phenotypically constrained, likely leading to significant underdiagnosis.
Cilia are complex molecular machines consisting of over a thousand proteins that contribute to their motility and function. To function properly, MCCs must:
- Generate over 100 centrioles that will become the basal bodies of their motile cilia.
- Dock those centrioles with the apical surface.
- Nucleate the proper number and length of cilia.
- Generate proper cilia motility.
- Coordinate the timing and orientation of cilia motility.
Defects in any of these steps will lead to a decrease in mucociliary flow yet the diagnosis of PCD has been primarily focused on cilia motility. We have developed the ciliated epithelia of the Xenopus embryonic skin as a powerful system to address numerous aspects of ciliary function. Importantly, we have developed quantifiable assays for each step of MCC formation and can identify the underlying cause of a decrease in mucociliary flow. Our combinatorial approach will facilitate a detailed analysis of multiple genes that are either linked to PCD without a clear mechanism or have only been predicted to cause PCD.
Project 2: Molecular regulation of radial intercalation
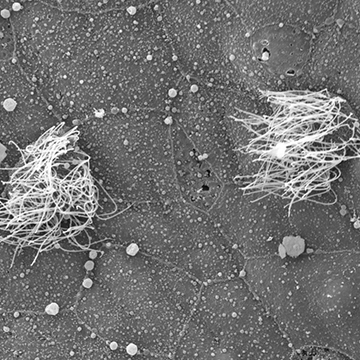 Multiciliated cells in the skin of Xenopus embryos initially differentiate in a distinct sub-layer of the epithelium and must undergo a short directed migration prior to intercalating into the outer epithelium. Our lab is exploiting this reiterated developmental process to study the molecular mechanisms by which cells migrate in a directed manner and how migrating cells penetrate through epithelial barriers. We have found that this process requires a delicate regulation of cytoskeletal dynamics. We are currently exploring numerous cytoskeletal regulators to understand how cells achieve directed movement and how these cells manipulate the junctional complexes of surrounding cells to facilitate their incorporation into the skin.
Multiciliated cells in the skin of Xenopus embryos initially differentiate in a distinct sub-layer of the epithelium and must undergo a short directed migration prior to intercalating into the outer epithelium. Our lab is exploiting this reiterated developmental process to study the molecular mechanisms by which cells migrate in a directed manner and how migrating cells penetrate through epithelial barriers. We have found that this process requires a delicate regulation of cytoskeletal dynamics. We are currently exploring numerous cytoskeletal regulators to understand how cells achieve directed movement and how these cells manipulate the junctional complexes of surrounding cells to facilitate their incorporation into the skin.
Project 3: Regulation of cell extrusion and macropinocytosis
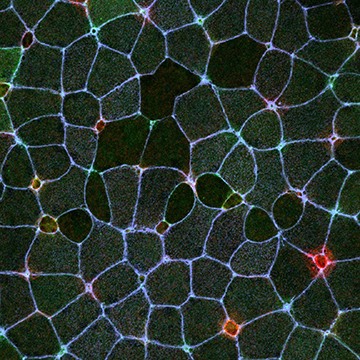 We have recently published that all MCCs undergo bi-directional cell extrusion. The first wave of basal cell extrusion is regulated via Notch signaling, and we will address the temporal control of this regulation which we hypothesize stems from a transcriptional shift in receptor subtype. Additionally, cells that evade Notch signaling are targeted for apical cell extrusion via a mechanosensory mechanism that we hypothesize is linked to cell crowding and the need to decrease apical tissue area. We will address the consequence of modulating cell number / type on the timing of apical extrusion. Using a long-term light-sheet imaging paradigm, we have identified numerous macropinocytotic events that increase as development progresses. Importantly, these events result in a significant loss of apical surface area, and we hypothesize that similar to apical cell extrusion, these events counter cell crowding by decreasing apical tissue area, but in a more subtle and less permanent manner. We will address both the regulation of these events and the functional consequence of inhibiting them, which we hypothesize will alter cell crowding thereby affecting cell extrusion.
We have recently published that all MCCs undergo bi-directional cell extrusion. The first wave of basal cell extrusion is regulated via Notch signaling, and we will address the temporal control of this regulation which we hypothesize stems from a transcriptional shift in receptor subtype. Additionally, cells that evade Notch signaling are targeted for apical cell extrusion via a mechanosensory mechanism that we hypothesize is linked to cell crowding and the need to decrease apical tissue area. We will address the consequence of modulating cell number / type on the timing of apical extrusion. Using a long-term light-sheet imaging paradigm, we have identified numerous macropinocytotic events that increase as development progresses. Importantly, these events result in a significant loss of apical surface area, and we hypothesize that similar to apical cell extrusion, these events counter cell crowding by decreasing apical tissue area, but in a more subtle and less permanent manner. We will address both the regulation of these events and the functional consequence of inhibiting them, which we hypothesize will alter cell crowding thereby affecting cell extrusion.