Determining how protein misfolding and aggregation results in cell death.
The Mazzulli Lab
The Mazzulli Lab is interested in determining how protein misfolding and amyloid formation results in cell death. Many chronic neurodegenerative disorders such as Parkinson’s disease, and Lewy body related dementias are characterized by the accumulation of misfolded proteins within the nervous system. We use patient neurons derived from induced pluripotent stem cells (iPSC) coupled with analytic biochemical techniques to study protein accumulation and lysosomal clearance mechanisms.
Proteotoxicity
Our goal is to understand the relationship between amyloid formation and neurotoxicity to uncover new therapeutic targets for neurodegenerative diseases
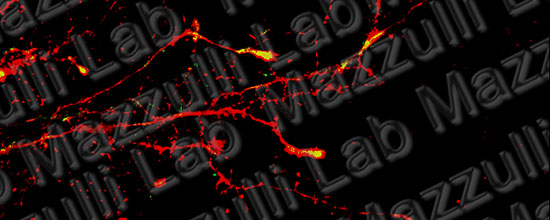
Pathways to the Lysosome
Discovering new pathways to improve lysosomal function in neurodegenerative proteinopathies
Lab Leadership

Joseph R Mazzulli, PhD
Associate Professor in the Ken and Ruth Davee Department of Neurology