About Nucleoskeletal Intermediate Filament Proteins: The Nuclear Lamins
Lamins are concentrated in the nuclear lamina, a structure that forms a molecular interface between the inner nuclear envelope membrane and chromatin. In lesser concentrations, the lamins are distributed throughout the nucleoplasm (see Figure below). In humans, there are two major types of lamins: A-type lamins (consisting of lamins A and C), found primarily in differentiated cells, and B-type lamins (lamins B1 and B2) that are found in all nucleated cells.
Lamin Protein Structure
Lamins have a central rod domain resembling that of cytoskeletal IF proteins, with the exception that there are an additional six heptads in coil 1B. In vitro studies have shown that dimers are the basic building blocks of higher order lamin structures and these dimers interact in a head-to-tail fashion to form protofilaments. Unlike cytoplasmic IF, lamins are not capable of assembling into 10nm diameter filaments in vitro. Until recently, little information was available regarding the precise types of structures lamins form within nuclei, but as described below recent cryo-EM studies are providing new insights into their in situ organization.
Function
Nuclear lamins are involved in a number of essential nuclear functions, including nuclear envelope assembly and disassembly during cell division, DNA synthesis, transcription, and apoptosis. Studies have shown that the addition of dominant-negative mutant lamins to Xenopus nuclei results in co-localization of PCNA and factor C (RFC), two known replication factors, with the lamin aggregates. With the breakdown of the lamina, it was also shown that DNA synthesis was inhibited of >95%, implying that lamins are integral to DNA synthesis. Lamins are also believed to regulate transcription; for example LA and LC bind to transcription factors such as pRB, which binds to LAP2. Disruption of lamin assembly results in significant inhibition of pol II activity and alteration in the splitting factors B” and Y12’s formations.
Diseases
Many interesting possibilities for additional lamin functions are revealed by the recent discoveries showing that many different mutations in human lamin A are associated with a remarkable array of diseases including Emery–Dreifuss muscular dystrophy (EDMD); dilated cardiomyopathy (DCM); familial partial lipodystrophy (FPLD); mandibuloacral dysplasia (MAD); autosomal recessive Charcot–Marie–Tooth disorder type 2 (AR-CMT2); limb girdle muscular dystrophy 1B (LGMD-1B) and premature aging diseases (Hutchinson-Gilford Progeria Syndrome (HGPS) and atypical forms of Werner’s Disease). The age of onset and the symptoms of these diseases vary over a wide range, but there are overlapping similarities, including muscle wasting, locomotory problems, fat redistribution, and cardiovascular problems. The nuclei in cells derived from patients with these “laminopathies” are frequently abnormal in shape and numerous components of these diseased nuclei are altered including nuclear pore complexes.
Some of Our Research on Nucleoskeletal IF Proteins
Structure & Organization of the Nuclear Lamins
With recent advances in high resolution light microscopy, including laser scanning confocal and structured illumination super-resolution microscopy, in conjunction with computational image analysis, we have been able to decipher the organization of the 4 lamin isoforms. The results show that each lamin isoform assembles into a distinct fiber meshwork (see Figure at the right) and that these separate networks have precise roles in maintaining the organization of the nuclear lamina. For the highest resolution analysis of nuclear lamins we have been collaborating with Dr. Ohad Medalia of the University of Zurich to use cryo-electron tomography to examine un-fixed specimens. The lamins exhibit a structure that is remarkably different from that of the canonical cytoplasmic IF: the lamins assemble into tetrameric filaments that are only 3.5 nm thick (see Figure below). Future studies will attempt to determine changes in organization that result from known human lamin mutations that result in disease such as muscular dystrophy and progeria. In addition, studying mouse models that lack some of the lamin isotypes should provide additional insights into lamin organization and function.
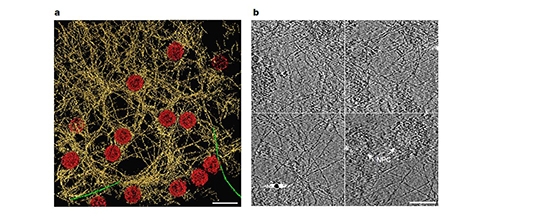
Pictured on the left: Architecture of the filamentous meshwork of the lamina of the mammalian cell nucleus. a, Rendered view of a representative 70 nm thick tomogram slice illustrates the putative lamin filaments (yellow), NPCs (red) and occasionally observed cytoplasmic actin filaments (green) on top of the nucleus (n = 55). Scale bar, 200 nm. b, Tomogram slices, 2 nm thick, from different regions of the nuclear lamina. Scale bar, 100 nm.
For additional basic information on nucleoskeletal intermediate filaments please watch this lecture given by Dr. Goldman: