Our Work
For the past 10 years, our work has been focussed on the role of tau in Alzheimer's disease (AD) and other taoupathies. AD, the most common cause of dementia among people age 65 and older, is characterized by the progressive and irreversible degeneration of neuronal processes and the consequent loss of synaptic connections. This disease has been studied from many angles including its etiology, diagnosis, potential treatments, and prognosis. As a result of these studies, much is known regarding the molecular composition of the senile plaques and neurofibrillary tangles, the AD hallmark lesions. Senile plaques are composed of extracellular deposits of Ab derived by proteolytic cleavage from the amyloid precursor protein. Neurofibrillary tangles, on the other hand, are intracellular bundles of self-assembled tau proteins. In the past decade, research has focused on a potential link between these two lesions. The data obtained support a key role for tau in the mechanisms leading to Ab-induced neurodegeneration in the central nervous system. Building upon work of many others, we have shown that neurons expressing either mouse or human tau degenerated in the presence of preaggregated Ab, while no signs of degeneration were detected in Ab-treated tau-depleted neurons. More recently, evidence has emerged linking tau cleavage with neuronal death. We have identified a 17 kDa tau fragment (tau45-230) that is the result of calpain cleavage. High levels of this fragment have been detected in cultured hippocampal neurons incubated in the presence of Ab as well as in brain samples obtained from AD animal model systems. In addition, the expression of this tau fragment in otherwise healthy hippocampal neurons induces progressive neuronal degeneration leading to cell death. Our results also showed enhanced neuronal death and synapse loss in transgenic mice expressing tau45-230. Ongoing research in our lab is focussd on elucidating the mechanisms underlying the toxic effects of tau45-230 in the context of neurodegenerative diseases.
Progressive degeneration in cultured hippocampal neurons obtained from transgenic tau45-230 mice
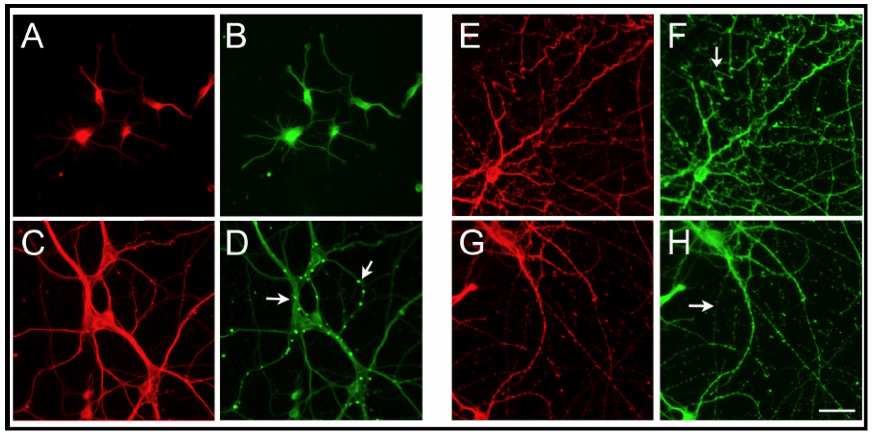
(A-H) Hippocampal neurons obtained from transgenic tau45-230 mice were kept in culture for 1 (A-D), 7 (E&F), 14 (G&H) and 21 (I&J) days. Neurons were fixed and immunostained using a tubulin (A,C, E, G & I) and our tau45-230 (B, F, H & J) antibodies. Tau45-230 immunoreactivity increased as the neurons developed in culture. Note the absence of immunoreactivity when the tau45-230 antibody was omitted (D) and the change in the distribution of tau45-230 from a punctate localization (arrow in F) to a more diffuse immunoreactive pattern after the first week in culture. Signs of progressive degeneration, including tortuose processes, were readily detected as early as 14 days in culture neurons (arrow in H). Complete degeneration of neuritic processes was evident in 21 days in culture hippocampal neurons (arrow in J). Scale bar: 20µm.