There are many unexplored questions in the growing field of autophagy, spanning from the molecular mechanisms of autophagy induction to the physiological roles of autophagy in health and disease. Our research interest focuses on the roles and mechanisms of autophagy in the regulation of metabolism and behavior and in the pathogenesis of related diseases, such as obesity and diabetes, neurodegeneration, and drug addiction. There are currently three major directions of study in our laboratory:
1. How autophagy regulates insulin sensitivity, energy metabolism, and exercise-induced metabolic benefits
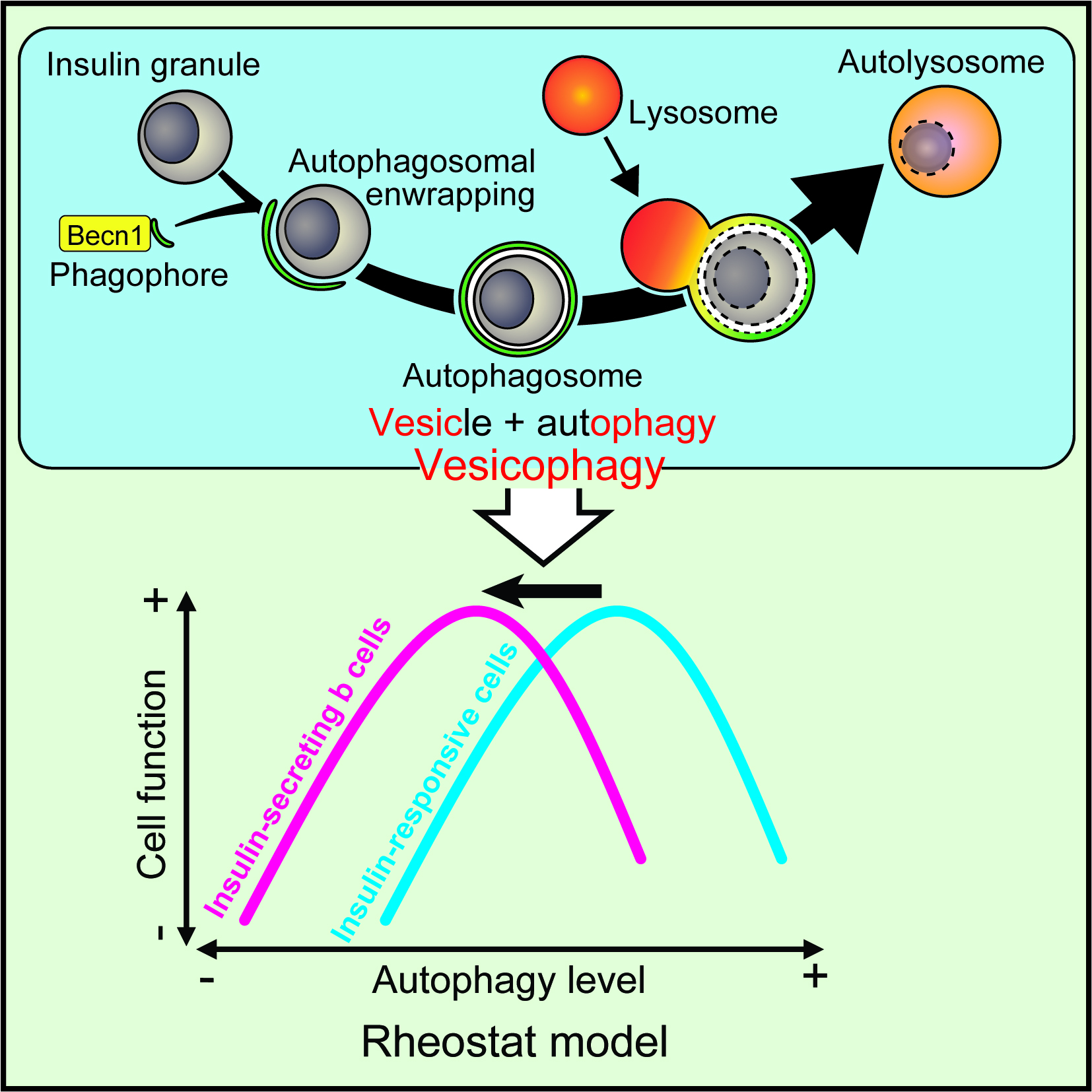
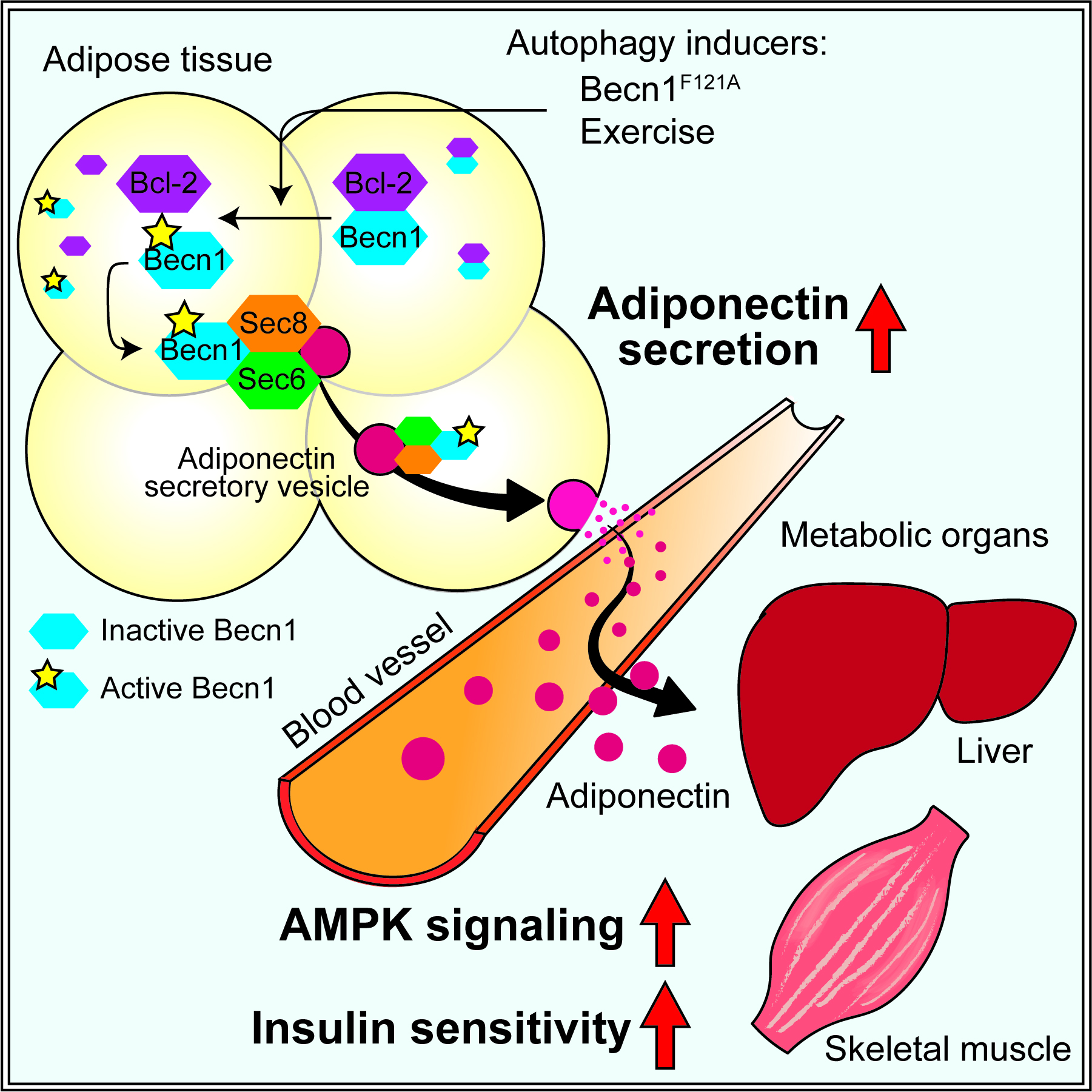
We aim to understand how autophagy regulates energy metabolism and insulin sensitivity. We generated and characterized a mouse model that shows high autophagy activity even under non-autophagy-inducing conditions. In these mice, we engineered a single mutation into a key autophagy gene Becn1, which disrupts an inhibitory binding and leads to constitutively active autophagy. We found that in response to high-fat diet challenge, Becn1-mediated autophagy hyperactivation increases insulin sensitivity by promoting adiponectin secretion from the adipose tissue, but decreases insulin secretion and storage via autophagic degradation of insulin granules, thereby demonstrating the differential roles of autophagy on metabolic regulation in insulin-responsive cells versus insulin-secreting β cells. In addition to genetic upregulation of autophagy, we revealed that pharmacological autophagy inducers (ML246 and Rg2), as well as a physiological autophagy inducer (physical exercise), also have beneficial effects on cognitive function and insulin sensitivity. We are continuing to investigate the mechanism and therapeutic potential of for autophagy activation in protecting against metabolic and age-related degenerative diseases.
2. How autophagy proteins regulate neuronal receptor metabolism and lead to behavioral output
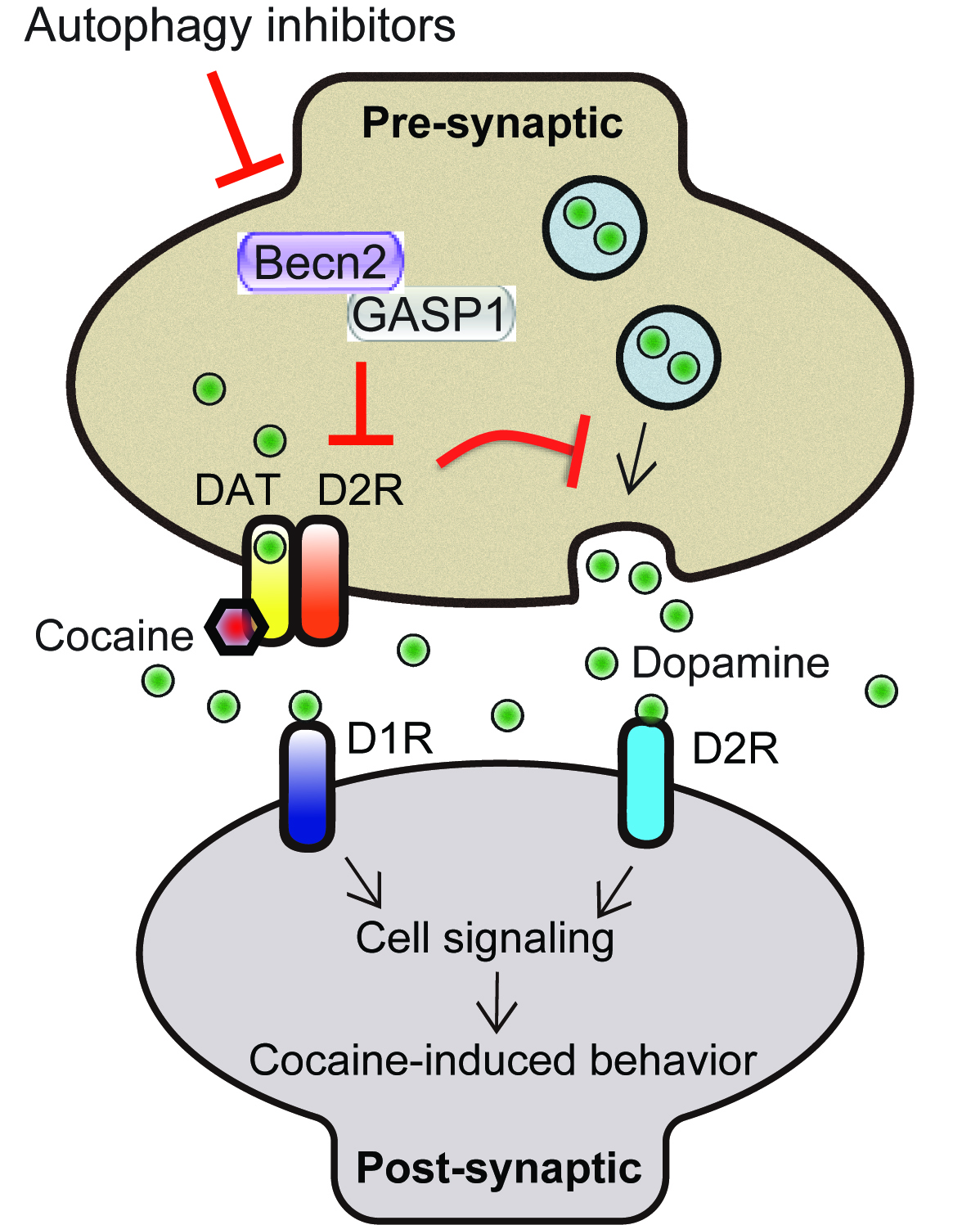
We are interested in understanding how the autophagy pathway regulates the behavioral responses to addictive drugs, such as cannabinoids and cocaine, which generate profound behavioral effects through activating cell-surface GPCRs (G protein-coupled receptors) in the brain. Although many efforts have been made to understand drug abuse and addiction, the pathogenic mechanisms of drug-related disorders are unresolved and effective treatments are yet to be discovered. We recently discovered that an autophagy-essential Beclin/Becn family member protein genetically links drug tolerance and addiction to the autophagy pathway by regulating GPCR catabolism. Thus, we are uncovering the genetic basis, the mechanism, and the therapeutic effect of the autophagy pathway in the regulation of drug reward and addiction using mouse models.
3. Autophagy in neuroinflammation and neurodegeneration
The accumulation of protein aggregates has been implicated in the pathogenesis of several neurodegenerative diseases. One approach by which these aggregates can be eliminated is through the activation of autophagy. We generated a variety of genetic tools, including mouse models with deficient or hyperactive autophagy, to study the cellular function and mechanism of autophagy in the clearance of aggregate-prone proteins and in the protection of cognitive behaviors. We are also studying the therapeutic potential of novel autophagy-inducing synthetic or natural compounds in the prevention of neuroinflammation using a mouse model of Alzheimer’s disease.