Research
Reactive oxygen species (ROS) is a collective term referring to short-lived species produced continuously by the body as by-products of the energetic metabolism. The majority of ROS behave as oxidants in biological settings, altering biomolecule structure and function. This leads to cumulative changes in cellular structures like membranes, the chromatin and organelles, forcing adaptations linked to disease onset and progression.
Key Findings
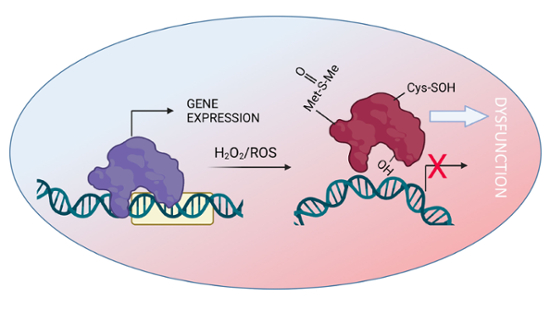
Our laboratory found that an increase in the production of ROS, such as those that occur during normal aging, changes how genes are spatially organized in the nucleus and what affects their function, reflecting changes to cells or phenotypes. Though ROS have been known for decades to directly damage DNA, causing strand breaks and mutations, our results indicate that levels insufficient to directly damage chromatin can nevertheless regulate its organization in ways that can trigger the onset and sustain the progression of disease.
Redox regulation of inflammatory transcription
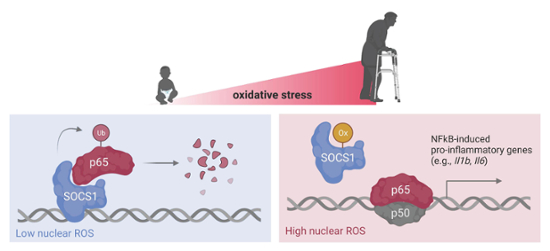
In this project, we explore how changes in the electrochemical state of the nucleus caused by an increase in steady-state ROS dysregulate inflammatory transcription leading to hyper-inflammatory states in macrophages. Macrophages are innate immune cells acting as first responders against pathogens such as viruses and bacteria. An exaggerated response to pathogens by macrophages sets up the entire immune system to overreact, leading to dangerous, life-threatening inflammation. In 2015, we found that the enzyme suppressor of cytokine signaling-1 (SOCS1), a negative regulator of the master inflammatory transcription factor nuclear factor kB, is oxidized and thereby inhibited by ROS. A transient inhibition by ROS allows NFkB to bind to and initiate the transcription of inflammatory genes necessary to control infection. However, it is critically important that inflammatory transcription by NFkB is shutdown by SOCS1 in order for healing to begin. Along these lines, we recently found that SOCS1 physically interacts with promoter regions of certain pro-inflammatory genes and that once removed it must be reloaded on to those loci to shut off transcription.
It is our hypothesis that more chronic elevations in the levels of nuclear ROS prevent the reloading of SOCS1 onto those genomic loci, in addition to suppressing its interaction with NFkB. This combination primes macrophages to assume hyper-inflammatory states upon activation and may contribute to some significant extent to the higher susceptibility of elderly patients to inflammatory tissue damage secondary to infection. Please visit our Publications page to learn more.
Electrochemical epigenetics
Most biological processes essential to life depend on biomolecules that work concertedly in order to provide energy, building blocks and other materials for cellular organization and function. The assembly of these biomolecules into complexes and their very functions depend on their ability to assume specific shapes and forms. The physicochemical environment where these molecules are inserted influences their shapes by modifying electrochemical charges, solubility or atomic composition. Intuitively, it is understandable that changes in the pH, viscosity, polarity of the media fundamentally determine the shapes a specific polymer (i.e. protein or DNA). Another physicochemical force of growing importance is the redox milieu. It has been reported that mitochondria and the nucleus are connected by channels through which different metabolites diffuse (Desai et. al., Sci Adv. 2020). As mitochondria age, the wear and tear of mitochondrial structures contribute to the decline of the energic metabolism, which is almost always associated with an increase in ROS production.
To study the impact of ROS on the regulation of gene expression, we combined methodologies to produce ROS directly and specifically in the cell nucleus with biosensors to accurately quantify them. Using these approaches, we found that transient elevations in the levels of nuclear ROS are sufficient and required to trigger epithelial-to-mesenchymal transition (EMT), a phenotypic transition of cancer cells linked to the development of treatment resistance and metastasis. By targeting nuclear ROS with genetically encoded tools, we drove established metastatic lesions into remission and resensitized chemoresistant persister cells to chemotherapy. Collaborations with the pharmaceutical industry are ongoing to develop new pharmacologic treatments based on these studies.
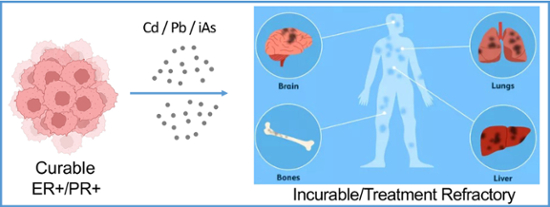
Metallic contaminants and breast cancer outcomes
Hundreds of millions of individuals worldwide live in areas affected by metal pollution. Incidents like the lead poisoning of drinking water in Flint, Michigan in 2015 or the chronic exposures to arsenic in densely populated areas in South and Southeast Asia have shown that the effects of metals on human health are undeniably serious and unequally impact poorer populations. In 2020, we reported that exposure to arsenic downregulates the expression of estrogen and progesterone receptors in breast cancer, leading to phenotypes that are significantly more challenging to treat (i.e. refractory and metastatic). We recently found that the observations made with arsenic are generalizable for cadmium and lead as well as their combinations at levels significantly lower than those recommended by world environmental health authorities. These studies led us to the discovery that heavy metals acting like mitochondrial poisons increase the levels of ROS production that inevitably reach the nucleus. In addition, we found that the progesterone receptor gene (PGR) is a redox-sensitive gene regulated by ROS. This finding extended research in the laboratory towards developing nuclear ROS scavengers to mitigate the detrimental effects of metal pollution on patients fighting breast cancer who may have worse prognosis and outcomes based on environmental health injustice and socio-economic disparities.
Ongoing Projects
Redox-sensitive switches controlling macrophage inflammatory function
This project explores molecular mechanisms linking changes to the metabolism of aging macrophages that lead to hyper-inflammatory responsiveness to pathogens. One recent observation that illustrates the impact of this project is the clearly heightened susceptibility of unvaccinated elderly populations to developing severe COVID-19. Our results indicate that ROS produced by mitochondria disables anti-inflammatory mechanisms critical for the transcriptional regulation of pro-inflammatory genes encoded in the nucleus.
Funding: National Institutes of Health, grants R01AI131267 and R01HL163820
Redox epigenetic reprogramming of the cancer cell transcriptome
This project investigates how changes to the electrochemical environment of the nucleus caused by an increase in the steady-state ROS changes chromatin organization structurally and epigenetically. Among our main findings is the observation that nuclear ROS are sufficient and required for epithelial to mesenchymal transition, a process involved in the development of treatment resistance and metastasis. By targeting nuclear ROS we were able to drive metastatic lesions into full remission in mice and re-sensitize chemoresistant cancer cells to treatment.
Funding: National Institutes of Health, grant R01CA216882
Heavy metal pollution and the evolution of breast cancer to hormone receptor negative subtypes
Hormone receptor (HR) status is a key prognostic indicator. HR+ tumors normally respond well to treatment and have excellent outcomes, while HR- tumors tend to fail treatment and metastasize. We found that lead, cadmium and arsenic at levels considerably lower than those recommended by the EPA downregulate HR in breast cancer, essentially converting HR+ breast cancer into HR-. Targeting nuclear ROS suppresses this process and may represent a novel short-term pharmacologic solution for low-income populations living in highly polluted areas who are therefore affected by environmental health injustice.