Our Work
The Abdel-Mohsen Lab integrates glycomics, immunology, virology and systems biology to explore how glycans influence immune function during chronic diseases and aging. Our research focuses on how glycan changes (on cells, in circulation and within the gut) impact immune surveillance, microbial balance and drive inflammaging.
Believing that glycans are central regulators of immunity, our lab has helped shape the emerging field of translational glycoimmunology. We utilize cutting-edge tools such as:
- Mass spectrometry-based glycomics.
- Glycan engineering.
- Lectin-based profiling.
- Multi-omics integration.
- Patient-derived biospecimens.
- Gut organoids.
- Humanized and transgenic mouse models.
Glycans, complex sugars found on the surface of cells and circulating proteins, play a vital role in immune regulation, microbial balance and inflammation. Once overlooked due to their complexity, glycans are now recognized as key players in disease and aging thanks to advanced glycomic technologies.
Our Research Framework
We follow a three-phase approach to uncover and translate glycan biology:
- Discovery
Identify disease-associated glycomic signatures using well-characterized human cohorts. - Characterization
Dissect mechanisms by which glycan changes influence immune function using in vitro assays and advanced in vivo models. - Development
Translate findings into new diagnostic tools and glyco-immune-based therapies for chronic disease and aging.
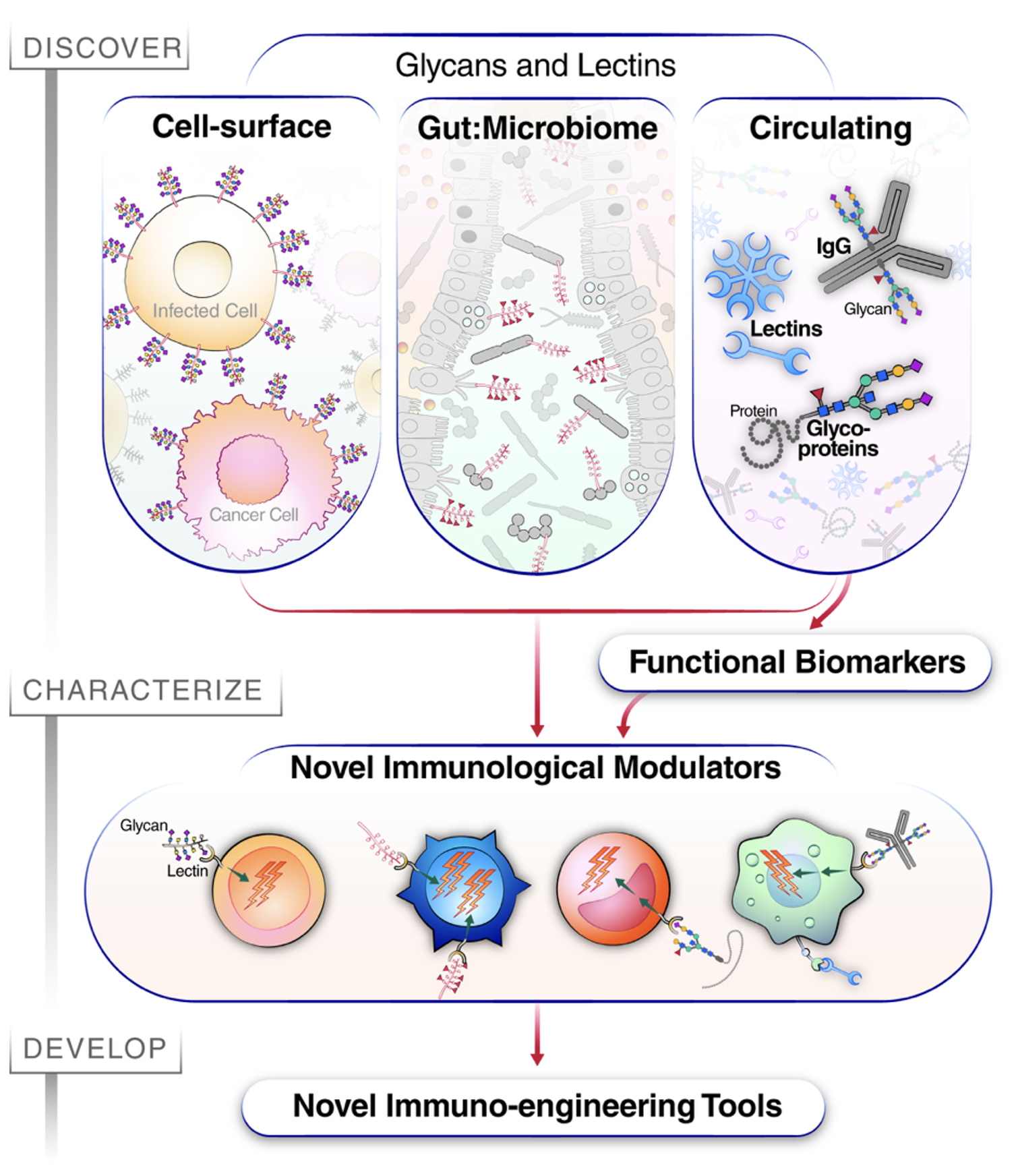
Research Areas
1. Glyco-Immune Checkpoints in Cancer and Viral Infections
Pathologic cells often remodel surface glycans to evade immune detection. By increasing specific glycan structures that bind to inhibitory lectins (e.g., Siglecs) on innate immune cells, these cells suppress immune attack.
We study these glyco-immune checkpoints in cancers (e.g., pancreatic cancer, glioblastoma) and chronic viral infections (e.g., HIV), developing therapies like Siglec-blocking antibodies and enzyme-conjugated antibodies to restore immune clearance.
2. Gut Glycome–Microbiome Interactions in Inflammation and Aging
The gut’s glycan landscape is a key interface with the microbiome. In aging and chronic infections, glycan disruption leads to microbial imbalance and systemic inflammation.
We investigate how intestinal glycosylation changes, often driven by senescence, depriving beneficial microbes of “intrinsic prebiotics.” Using human samples, organoids and animal models, we aim to develop glycan-targeted dietary supplements to restore gut health and prevent inflammation-driven complications.
3. Circulating Glycans as Biomarkers and Drivers of Inflammaging
Glycans on circulating glycoproteins, especially antibodies, regulate inflammation and reflect biological aging.
We study how anti-inflammatory sugars (e.g., sialic acid, galactose) decline with age while pro-inflammatory glycans accumulate, contributing to inflammaging. Our goal is to identify glycan-based biomarkers of healthspan and disease risk, and explore glycoengineering strategies to improve immune function and reduce age-related comorbidities.