The Function of CD95 as a Tumor Promotor
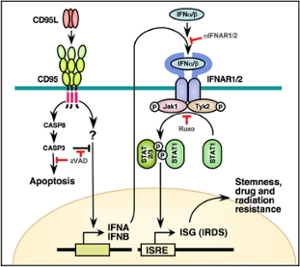 The most highly CD95 apoptosis sensitive cells with the highest CD95 expression are cancer cell lines. That is incompatible with the established function of CD95 as a tumor suppressor as it represents one of two ways for a cytotoxic killer cell to kill a cancer cells. In 2004, the Peter Lab was the first to report that stimulation of CD95 on either apoptosis resistant cancer cells or even on cells that were sensitive in vitro when stimulated with a physiologically relevant CD95 ligand responded with an increase in motility and invasiveness through activating a number of prosurvival pathways (1). They subsequently found that CD95 is barely if ever deleted in both alleles. In all cases of heterozygous deletion while apoptosis was impaired nonapoptotic signaling (such as NF-κB activation) was unaffected (2). These data were of fundamental importance considering that death ligands such as CD95L and certainly TRAIL have been in clinical development for the treatment of cancer. To raise awareness on the subject Dr. Peter wrote a review on the nonapoptotic activities of CD95 and together with 13 other colleagues (3). In order to address the question on the role of CD95 in cancer the Peter group decided to generate tissue specific knock-out mice lacking CD95 expression in the ovaries or the liver. These mice were subjected to appropriate tumor models and in both cases mice lacking CD95 either formed reduced cancer (chemically induced liver cancer) or almost no cancer at all (endometrioid ovarian cancer). They concluded that cancer cells require CD95 for their growth (4). More recently, CD95 was identified as being required for the generation and maintenance of cancer stem cells in a number of different cancers (4). Most recently the Peter Lab demonstrated that this activity of CD95 acts through inducing type I interferons and activation of STAT1 (6).
The most highly CD95 apoptosis sensitive cells with the highest CD95 expression are cancer cell lines. That is incompatible with the established function of CD95 as a tumor suppressor as it represents one of two ways for a cytotoxic killer cell to kill a cancer cells. In 2004, the Peter Lab was the first to report that stimulation of CD95 on either apoptosis resistant cancer cells or even on cells that were sensitive in vitro when stimulated with a physiologically relevant CD95 ligand responded with an increase in motility and invasiveness through activating a number of prosurvival pathways (1). They subsequently found that CD95 is barely if ever deleted in both alleles. In all cases of heterozygous deletion while apoptosis was impaired nonapoptotic signaling (such as NF-κB activation) was unaffected (2). These data were of fundamental importance considering that death ligands such as CD95L and certainly TRAIL have been in clinical development for the treatment of cancer. To raise awareness on the subject Dr. Peter wrote a review on the nonapoptotic activities of CD95 and together with 13 other colleagues (3). In order to address the question on the role of CD95 in cancer the Peter group decided to generate tissue specific knock-out mice lacking CD95 expression in the ovaries or the liver. These mice were subjected to appropriate tumor models and in both cases mice lacking CD95 either formed reduced cancer (chemically induced liver cancer) or almost no cancer at all (endometrioid ovarian cancer). They concluded that cancer cells require CD95 for their growth (4). More recently, CD95 was identified as being required for the generation and maintenance of cancer stem cells in a number of different cancers (4). Most recently the Peter Lab demonstrated that this activity of CD95 acts through inducing type I interferons and activation of STAT1 (6).
References
- Barnhart, B.C., Legembre, P., Pietras, E., Bubici, C., Franzoso, G. and Peter, M.E. (2004) CD95 ligand induces motility and invasiveness of apoptosis resistant tumor cells. EMBO J. 23, 3175-3185.
- Legembre, P., Barnhart, B.C., Zheng, L., Vijayan, S., Straus, S.E., Puck, J., Dale, J.K., Lenardo, M. and Peter, M.E. (2004) Induction of apoptosis and activation of NF-κB by CD95 require different signaling thresholds. EMBO Reports, 5, 1084-1089.
- Peter, M.E., Budd, R.C., Desbarats, J., Hedrick, S.M., Hueber, A.-O., Newell, M.K., Owen, L.B., Pope, R.M., Tschopp, J., Wajant, H., Wallach, D., Wiltrout, R.H., Zörnig, M. and Lynch, D.H. (2007) The CD95 receptor: Apoptosis revisited. Cell, 129, 447-450.
- Chen, L., Park, S.M., Tumanov, A., Hau, A., Sawada, K., Feig, C., Turner, J., Fu, Y.X., Romeo, I., Lengyel, E. and Peter, M.E. (2010) CD95 promotes tumour growth. Nature, 465, 492-496.
- Ceppi, P., Hadji, A., Kohlhapp, F., Pattanayak, A., Hau, A., Liu, X., Liu, H., Murmann, A.E. and Peter, M.E. (2014) CD95 and CD95L promote and protect cancer stem cells. Nature Commun, 5:5238.
- Qadir, A.S., Ceppi, P., Brockway, S., Law, C., Mu, L., Khodarev, N.N., Kim, J., Zhao, J.C., Putzbach, W., Murmann, A.E., Chen, Z., Chen, W., Liu, X., Salomon, A.R., Liu, H., Weichselbaum, R.R., Yu, J., and Peter, M.E. (2017) CD95/CD95 increases stemness in cancer cells by inducing a STAT1 dependent type I interferon response. Cell Reports, 18, 2373–2386.