DISE - A Novel Concept for Cancer Therapy
The Peter Lab recently reported that si- and shRNAs derived from CD95, CD95L (1) and other genes in the human genome (2) kill cancer cells through RNAi by targeting a network of critical survival genes (1). DISE (death induced by survival gene elimination) was found to involve simultaneous activation of multiple cell death pathways, and cancer cells have a hard time developing resistance to this form of cell death (3,4). DISE was found to preferentially affect transformed cells (3) and among them cancer stem cells (5).
The following model represents the current view on how DISE works:
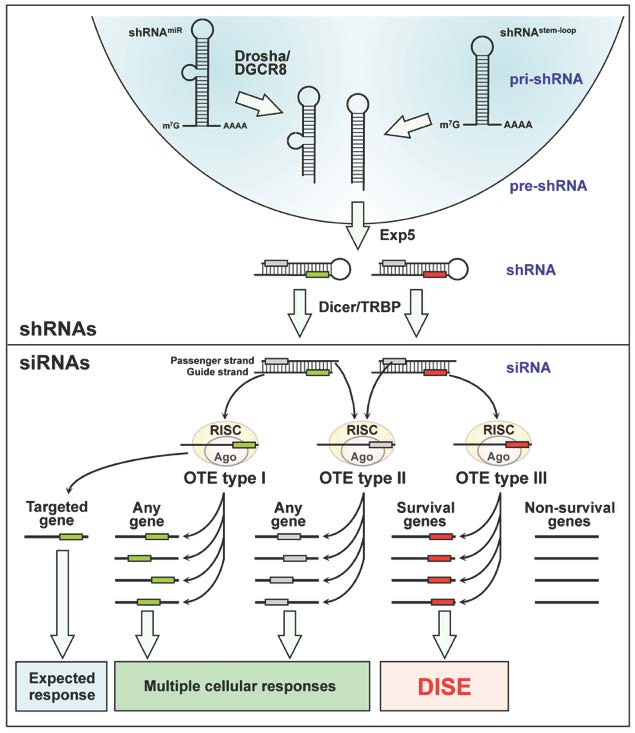
shRNAs, introduced most often in the form of a lentiviral vector and inserted into the genome, are transcribed by either RNA Pol II or III. They can either be simple stem loop shRNAs (shRNAstem-loop) or miRNA-based next generation shRNAs (shRNAmiR), which require Drosha/DGCR8 processing before they are exported into the cytosol by exportin 5 (Exp5). Each shRNA contains a sense/passenger strand and a complimentary antisense/guide strand. Dicer/TRBP trims these duplexes, thereby removing the loop region. Both trimmed shRNAs and exogenous and endogenous siRNAs are loaded into the RNA induced silencing complex (RISC) with their Argonaut proteins (Ago). Similar to miRNAs, positions 2-7 (the seed sequence) of the loaded guide strand greatly determines what target mRNA is silenced through complementarity of the seed sequence, mostly to the 3'UTR of the targeted mRNAs. In addition to the silencing of the mRNA that the siRNA was designed to target, siRNAs show three types of sequence-specific off target effects (OTE). OTE type I: The guide strand gets properly loaded into the RISC but its seed sequence (green box) targets other genes expressed in treated cells that show partial complementarity to the siRNA. Because this type of OTE can affect any type of gene, the resulting cellular responses are multiple (including different forms of cell death). OTE type II: the passenger strand of the siRNA, rather than the guide strand, is loaded into the RISC. Position 2-7/8 at the 5' end of the passenger strand (grey box) becomes the seed sequence, and targeting of mRNAs that show complementarity to the passenger strand seed sequence again results in multiple cellular responses. OTE type III: Certain seed sequences in the guide strand of specialized RNAs (red box) preferentially target seed matches present in the 3'UTR of survival genes and not non-survival genes. These siRNAs coevolved with sequences present in the 3'UTRs of certain survival genes. This OTE results in death of the treated cells called DISE.
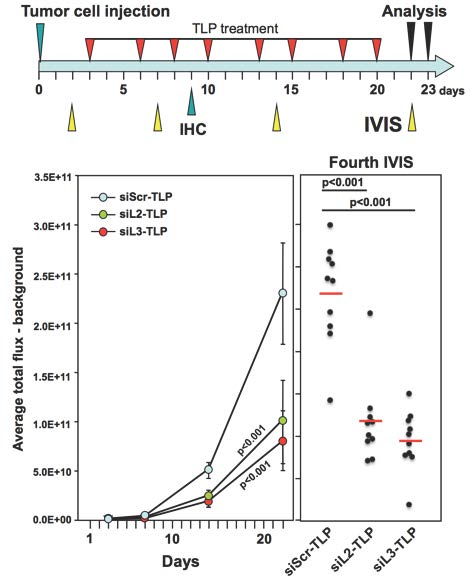 The Peter Lab has now explored how inducing DISE may be a novel form of cancer therapy (6). They first demonstrated that DISE induction works in an in vivo model whereby mice harbor xenografted ovarian cancer cells expressing a CD95L derived shRNA (shL3). They then delivered two siRNAs derived from CD95L (siL2 and siL3) to mice with ovarian cancer xenografts using a nanoparticle platform demonstrated previously to deliver siRNA in vivo (see Figure). Templated lipoprotein particles (TLP) stabilize siRNA and are dependent upon SR-B1 expression for efficient siRNA delivery. The TLPs were delivered i.p., taken up by the tumor cells, and acted through canonical RNAi, substantially reducing tumor growth by inducing DISE. The in vivo study was terminated once the control treated mice showed signs of discomfort due to large tumors and/or ascites formation. The remaining tumor cells from the nanoparticle delivered siL3 group were resected, cultured, and transfected with siL3 in culture using a commercially available cationic lipid-based transfection reagent in order to determine if the cells became insensitive to DISE. In this context, the tumor cells were still fully susceptible to DISE, suggesting that treatment optimization (i.e. dose and time) may allow for full eradication of tumor growth. Most importantly, treated mice did not show signs of toxicity. Collectively, these data demonstrate that DISE induction is a promising new approach for treating cancer.
The Peter Lab has now explored how inducing DISE may be a novel form of cancer therapy (6). They first demonstrated that DISE induction works in an in vivo model whereby mice harbor xenografted ovarian cancer cells expressing a CD95L derived shRNA (shL3). They then delivered two siRNAs derived from CD95L (siL2 and siL3) to mice with ovarian cancer xenografts using a nanoparticle platform demonstrated previously to deliver siRNA in vivo (see Figure). Templated lipoprotein particles (TLP) stabilize siRNA and are dependent upon SR-B1 expression for efficient siRNA delivery. The TLPs were delivered i.p., taken up by the tumor cells, and acted through canonical RNAi, substantially reducing tumor growth by inducing DISE. The in vivo study was terminated once the control treated mice showed signs of discomfort due to large tumors and/or ascites formation. The remaining tumor cells from the nanoparticle delivered siL3 group were resected, cultured, and transfected with siL3 in culture using a commercially available cationic lipid-based transfection reagent in order to determine if the cells became insensitive to DISE. In this context, the tumor cells were still fully susceptible to DISE, suggesting that treatment optimization (i.e. dose and time) may allow for full eradication of tumor growth. Most importantly, treated mice did not show signs of toxicity. Collectively, these data demonstrate that DISE induction is a promising new approach for treating cancer.
References
- Putzbach, W., Gao, Q.Q., Patel, M., van Dongen, S., Haluck-Kangas, A., Sarshad, A.A., Bartom, E., Kim, K.Y., Scholtens, D.M., Hafner, M., Zhao, J.C., Murmann, A.E., and Peter, M.E. (2017) Many si/shRNAs can kill cancer cells by targeting multiple survival genes through an off-target mechanism. eLife, 6, e29702.
- Patel, M. and Peter, M.E. (2017) Identification of DISE-inducing shRNAs by monitoring cellular responses. Cell Cycle, 17, 506-514.
- Hadji, H., Ceppi, P., Murmann, A.E., Brockway, S., A., Pattanayak, A., Bhinder, B., Hau, H., De Chant, S., Parimi, V., Kolesza, P., Richards, J., Chandel, N., Djaballah, H. and Peter, M.E. (2014) Death induced by CD95 or CD95 ligand elimination. Cell Reports 5, 208-222.
- Putzbach, W., Gao, Q.Q., Patel, M. (2018), Haluck-Kangas, A., Murmann, A.E. and Peter, M.E. DISE - An RNAi Off-Target Effect that Kills Cancer Cells. Trends in Cancer, 4, 10-16.
- Ceppi, P., Hadji, A., Kohlhapp, F., Pattanayak, A., Hau, A., Liu, X., Liu, H., Murmann, A.E. and Peter, M.E. (2014) CD95 and CD95L promote and protect cancer stem cells. Nature Commun, 5:5238.
- Murmann, A.E., McMahon, K., Haluck-Kangas, A., Ravindran, N., Patel, M., Law, C., Brockway, S., Wei, J.J., Thaxton, C.S., and Peter, M.E. (2017) Induction of DISE in ovarian cancer cells in vivo. Oncotarget - Priority Report, 8, 84643-84658.