Research
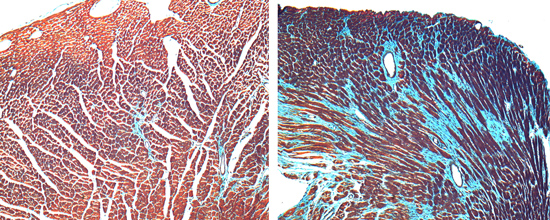
S1P1 signaling in cardiac development
Sphingosine 1-phosphate (S1P) is a bioactive ligand that binds to one of five G protein-coupled receptors (GPCRs). The S1P receptor S1P1 is highly expressed in endothelium, the central nervous system, lymphocytes, and the heart. S1P1 plays an unexpected role in cardiac development by promoting proliferation and cardiomyocye alignment in the compact layer of the developing heart; in the absence of cardiomyocyte S1P1, mice develop ventricular noncompation. We use the mouse model system, cell culture, and molecular biology techniques to ask the following questions: (1) What is the molecular mechanism that links the S1P1 receptor to the cardiomyocyte proliferation program and compaction? (2) How does S1P1 signaling direct or permit cardiomyocyte alignment? (3) Can S1P1 participate in a mechanosensory pathway in cardiomyocytes?
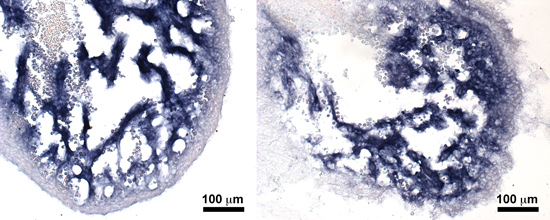
S1P1 signaling in cardiomyocyte maintenance and cardiac fibrosis
The loss of S1P1 in adult cardiomyocytes lowers the threshold for cardiac fibrosis in the setting of pathological stress. Interestingly, absence of S1P1 does not decrease left ventricular systolic (pumping) function. The combination of cardiac fibrosis and normal systolic function suggests the possibility of heart failure with preserved ejection fraction (HFpEF, also known as diastolic heart failure). No effective treatments for cardiac fibrosis or HFpEP currently exist, and therefore cardiomyocyte-specific loss of S1P1 may represent a good model system for potential treatments. We use mouse models, echocardiographic and microscopic imaging, cell culture and molecular biology techniques to address several questions: (1) What are the molecular mechanisms by which S1P1 protects the heart against fibrosis? (2) Does S1P1 mediate crosstalk between the cardiomyocytes and cardiac fibroblasts or cardiac endothelial cells? (3) Do S1P1 cardiomyocyte-null mice develop echocardiographic features of HFpEF? (4) what is the source of S1P for adult cardiomyocytes?