Disordered Proteostasis as a Driver of Disease in the Aging Lung: Endocrine Signaling of Proteostatic Stress in the Aging Lung
Influenza A pneumonia is a common cause of death from an infectious agent; it disproportionately affects the elderly in whom it is more severe. While the lung epithelium is the primary target of the influenza A virus (IAV) infection, the resulting illness is a multisystem disease and death is often attributable to development of the multiple organ dysfunction syndrome (MODS). Skeletal muscle weakness is a clinically important manifestation of MODS and is present in half of patients admitted to the intensive care unit with respiratory distress. 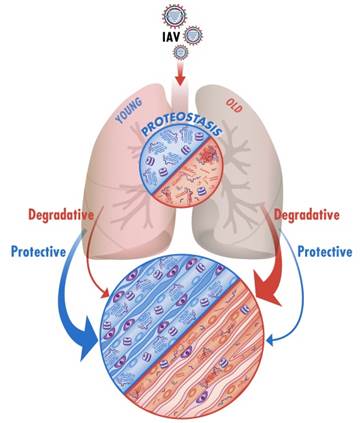 Skeletal muscle weakness can delay the discontinuation of mechanical ventilation and, even in patients who survive, persists for years after hospital discharge impairing quality of life. Older patients with IAV infection have higher rates of respiratory distress and are at increased risk for developing skeletal muscle dysfunction. An expanding body of literature reports that skeletal muscle dysfunction develops very soon after the onset of critical illness, suggesting it is triggered by an active signaling process. In preliminary experiments, we found that aged mice develop more severe and prolonged skeletal muscle dysfunction during IAV infection. Proteostasis is at the center of protein function and encompasses all cellular processes that regulate protein folding, misfolding, unfolding, degradation and repair. Our studies suggest that endocrine signals from the injured lung during IAV infection can disrupt skeletal muscle proteostasis pathways and contribute to skeletal muscle dysfunction. We are also finding that the slower recovery of the skeletal muscle function in aged mice during IAV pneumonia is the consequence of diminished proteostatic reserve in cells responsible for regenerating the damaged skeletal muscle. Interestingly, our preliminary imaging and proteomic data suggest that signals from the lung that induce muscle degradation are counterbalanced by a robust chaperone response to preserve proteostasis in young but not old animals. As such, we hypothesize that the increased severity of the skeletal muscle dysfunction in aged individuals after IAV infection is the consequence of increased degradative signals from the proteostatically challenged injured lung, a diminished proteostatic reserve in the skeletal muscle cells.
Skeletal muscle weakness can delay the discontinuation of mechanical ventilation and, even in patients who survive, persists for years after hospital discharge impairing quality of life. Older patients with IAV infection have higher rates of respiratory distress and are at increased risk for developing skeletal muscle dysfunction. An expanding body of literature reports that skeletal muscle dysfunction develops very soon after the onset of critical illness, suggesting it is triggered by an active signaling process. In preliminary experiments, we found that aged mice develop more severe and prolonged skeletal muscle dysfunction during IAV infection. Proteostasis is at the center of protein function and encompasses all cellular processes that regulate protein folding, misfolding, unfolding, degradation and repair. Our studies suggest that endocrine signals from the injured lung during IAV infection can disrupt skeletal muscle proteostasis pathways and contribute to skeletal muscle dysfunction. We are also finding that the slower recovery of the skeletal muscle function in aged mice during IAV pneumonia is the consequence of diminished proteostatic reserve in cells responsible for regenerating the damaged skeletal muscle. Interestingly, our preliminary imaging and proteomic data suggest that signals from the lung that induce muscle degradation are counterbalanced by a robust chaperone response to preserve proteostasis in young but not old animals. As such, we hypothesize that the increased severity of the skeletal muscle dysfunction in aged individuals after IAV infection is the consequence of increased degradative signals from the proteostatically challenged injured lung, a diminished proteostatic reserve in the skeletal muscle cells.