Motor Cortical Representation of Reaching and Grasping
Motor Learning in M1 and PMD
The motor system has an impressive ability to learn new skills and adapt movements to new environments. Mastery of a skill like playing the violin causes permanent changes in the brain areas controlling movement [1]. Yet, the motor system is also adept at learning skills on a much shorter time scale, such as learning to wield a new tool. This short-term motor learning process has been studied in humans using predictable force perturbations applied to the arm during reaching [2,3].
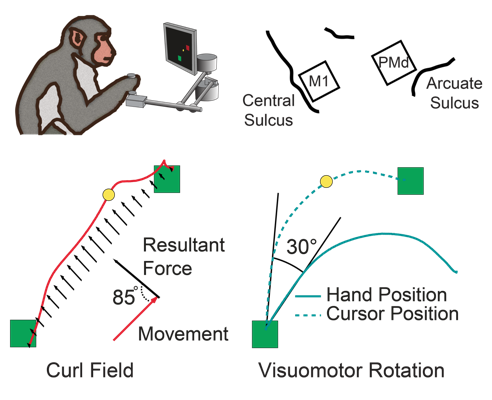
Over the course of ~100 reaches, research subjects learn to compensate for the perturbations to restore normal looking movements. Two common perturbations in these experiments are a "visuomotor rotation" (VR) [4], which offsets the visual feedback received about the movement, and the "curl field" (CF), a velocity-dependent force applied orthogonally to the hand movement direction (Figure 1).
We hypothesize that adaptation to these perturbations is mediated by altering the mapping
between vision-centric planning coordinates and muscle-centric movement coordinates believed
to occur in the motor cortices. We have previously shown that the relationship between the
activity of neurons in primary motor cortex (M1) and the dynamics of movement remain fixed
during curl field learning and thus cannot account for the adapted behavior [5]. Instead,
learning may be mediated by altered recruitment of M1 neurons by upstream brain areas. We
are testing this hypothesis by recording simultaneously from populations of neurons in M1
and dorsal premotor cortex (PMd). PMd plays a significant role in movement planning and is
a strong source of inputs to M1.
We compared the activity of neurons in these two areas as
monkeys adapt to either VR or CF perturbations, and found that single cells in both M1 and
PMd apparently maintain fixed relationships with movement covariates, either force or
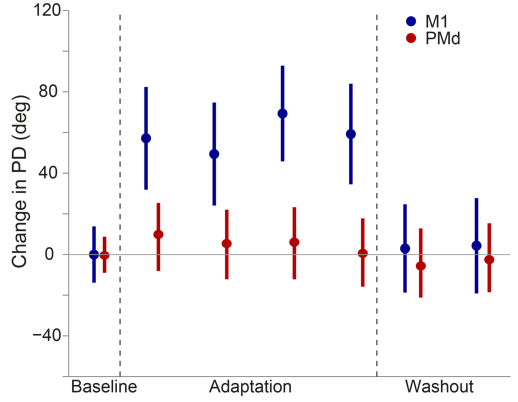
movement. In the curl field, for example, the "preferred direction" of M1 cells immediately
rotate when the curl field is applied (Figure 2, blue) while PMd cells show no change at
all (Figure 2, red). In neither area, is there a progressive change in tuning that can
explain the slow behavioral adaptation. These results suggest that learning these
perturbations does not result from changes within these networks. We are currently
focusing on population-level approaches to understand how these brain areas work together
to drive behavioral adaptation without changing the representations of single neurons.
Through these experiments, we hope to shed light on the cortical mechanisms underlying
motor learning, as well the functions of both M1 and PMd in coordinating movement.
↓ Jump to References
Neural Representation of Uncertainty in Reach Planning
Every movement we make represents just one of many possible actions. It is an open question
as to how the brain makes these choices, but it seems likely that the dorsal premotor cortex
(PMd) is involved. When presented with two discrete reach targets, PMd appears to represent
both options until one is shown to be the superior choice [6]. This has led some to speculate
that motor decisions arise from a neural competition between movement plans within PMd [7-9].
However, it is not clear how well these interpretations extend beyond the multiple choice
task.
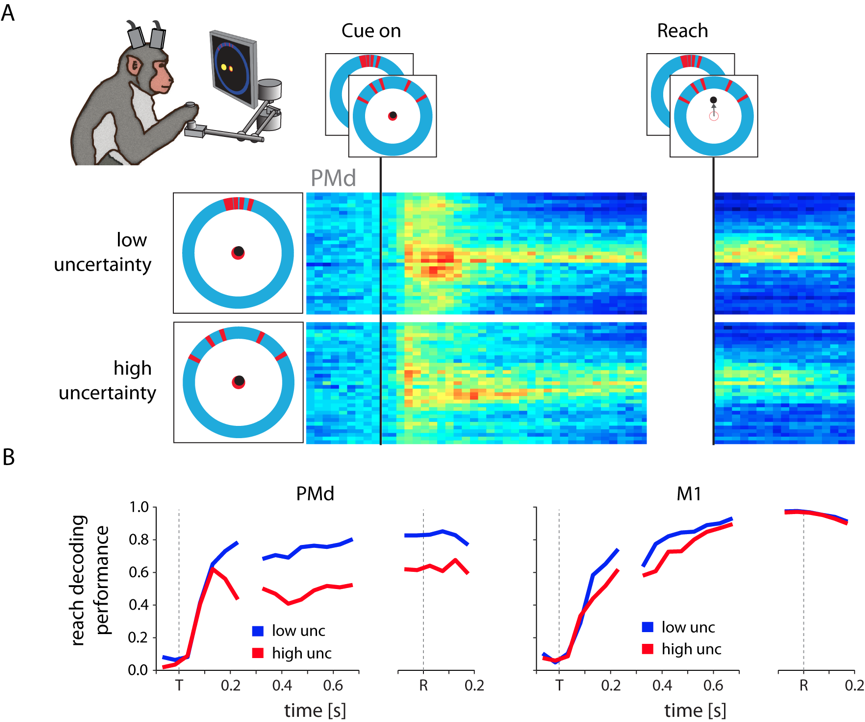
To probe the role of PMd in situations without clear targets, we performed an experiment in
which monkeys estimated the location of ambiguous targets represented by noisy visual cues.
We found that the planning-related activity in PMd changed significantly with the noisiness
of the cue, and that it appeared to reflect the monkey's subjective feeling of uncertainty
about his decision [10]. Interestingly, these effects persisted even after a decision had been
made (i.e., once a reach in a particular direction had started), an observation that appears
to be different from the multiple choice condition. We are currently performing additional
experiments to better understand the role of PMd in processing uncertainty and indecision
during motor planning.
↓ Jump to References
Neural Population Activity Patterns During the Control of Movement
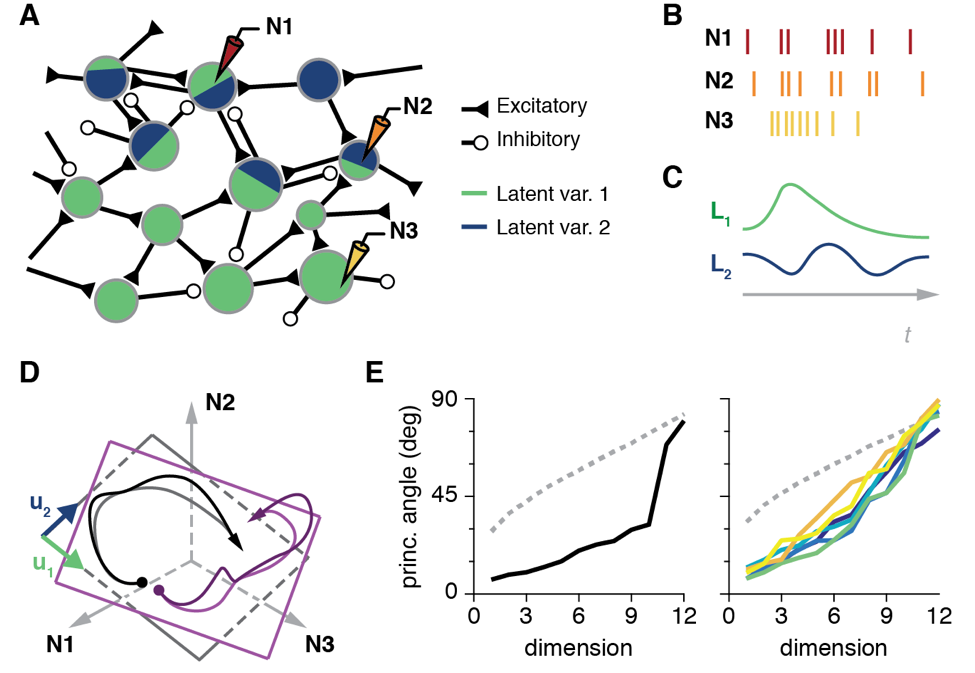
Both humans and monkeys are able to perform a rich repertoire of movements by controlling the activation of the complex musculature of the arm and the hand. The movement planning problem is formidable, as the nervous system needs to generate the control signals to perform such diverse actions as finely manipulating a delicate object, or swinging a heavy tool. Primary motor cortex (M1) is one important area involved in limb motor control. For several decades, the neural control of movement has been studied one neuron at a time, using individual electrodes carefully placed in M1 while monkeys made repeated, simple movements [11]. While these studies have been informative, revealing much of what we know of the characteristics of the movement related signals in the brain, they can say very little about the detailed interactions within the networks of neurons that actually generate these signals.
To understand these mechanisms, we identified the "neural modes," the dominant activity patterns in the population of tens to hundreds of neurons we record from [12]. To this end, we used dimensionality reduction techniques (Cunningham & Yu, 2014), mathematical methods that identify the dominant patterns of covariance across neurons that explain most of the population activity (Fig. 4A-C). Remarkably, for our standard lab tasks, few neural modes (typically less than ten) explain most of the neural population activity [13].
Geometrically, those few neural modes define a manifold, a low-dimensional surface embedded in the full "neural space" whose axes are the activity of each individual neuron (Fig. 1D). We compared the geometry of the manifolds from different tasks (Fig 4D), and observed that they were very close to each other (Fig. 4E). This suggests that the neural activity driving the execution of different behaviors lives in a similar portion of the neural space. We also found that time-varying activation of those neural modes is quite similar across tasks, although there are also task-specific population neural modes.
We are currently trying to elucidate the relationship between these neural activity patterns and the commands to the muscles. Our first results indicate that the muscle activation patterns for different tasks are generated by combining task-specific and task-independent neural population activity patterns. We are also performing experiments that aim to map the neural manifold during free movement, while we record data continuously for many hours. Our ultimate goals are to understand: 1) the similarity of this neural manifold across tasks, and its stability over time, 2) how the neural manifold is sculpted by learning, and 3) how the activity within the manifold generates the muscle activation patterns that ultimately drive behavior.
References
[1] Elbert, T., Pantev, C., Wienbruch, C., Rockstroh, B., & Taub, E. (1995). Increased cortical representation of the fingers of the left hand in string players. Science, 270(5234), 305-7.
[2] Lackner, J. R., & Dizio, P. (1994). Rapid adaptation to Coriolis force perturbations of arm trajectory. Journal of Neurophysiology, 72(1), 299-313.
[3] Shadmehr, R., & Mussa-Ivaldi, F. A. (1994). Adaptive representation of dynamics during learning of a motor task. J Neurosci 14(5 Pt 2), 3208-24.
[4] Martin, T. a, Keating, J. G., Goodkin, H. P., Bastian, a J., & Thach, W. T. (1996). Throwing while looking through prisms. I. Focal olivocerebellar lesions impair adaptation. Brain, 119 ( Pt 4, 1183-98.
[5] Cherian, A., Fernandes, H. L., & Miller, L. E. (2013). Primary motor cortical discharge during force field adaptation reflects muscle-like dynamics. Journal of Neurophysiology, 110(3), 768-83.
[6] Cisek, P. & Kalaska, J. F. Neural correlates of reaching decisions in dorsal premotor cortex: Specification of multiple direction choices and final selection of action. Neuron 45, 801-814 (2005).
[7] Klaes, C., Westendorff, S., Chakrabarti, S. & Gail, A. Choosing Goals, Not Rules: Deciding among Rule-Based Action Plans. Neuron 70, 536-548 (2011).
[8] Coallier, E., Michelet, T. & Kalaska, J. F. Dorsal premotor cortex: neural correlates of reach target decisions based on a color-location matching rule and conflicting sensory evidence. J. Neurophysiol. jn.00166.2014 (2015). doi:10.1152/jn.00166.2014
[9] Pastor-Bernier, A. & Cisek, P. Neural Correlates of Biased Competition in Premotor Cortex. J. Neurosci. 31, 7083-7088 (2011).
[10] Dekleva, B. M., Ramkumar, P., Wanda, P. A., Kording, K. P. & Miller, L. E. Uncertainty leads to persistent effects on reach representations in dorsal premotor cortex. Elife 5, 1-24 (2016).
[11] E. V Evarts, "Relation of pyramidal tract activity to force exerted during voluntary movement.," J. Neurophysiol., vol. 31, no. 1, pp. 14-27, 1968.
[12] J. A. Gallego, M. G. Perich, L. E. Miller and S. A. Solla, "Neural manifolds for the control of movement," Neuron, under review.
[13] J. P. Cunningham and B. M. Yu, "Dimensionality reduction for large-scale neural recordings," Nat. Neurosci., vol. 17, no. 11, pp. 1500-1509, 2014.
[14] M. M. Churchland, J. P. Cunningham, M. T. Kaufman, S. I. Ryu, and K. V. Shenoy, "Cortical Preparatory Activity: Representation of Movement or First Cog in a Dynamical Machine?," Neuron, vol. 68, no. 3, pp. 387-400, 2010.
[15] P. T. Sadtler, K. M. Quick, M. D. Golub, S. M. Chase, S. I. Ryu, E. C. Tyler-Kabara, B. M. Yu, and A. P. Batista, "Neural constraints on learning.," Nature, vol. 512, no. 7515, pp. 423-6, Aug. 2014.