Research
Ischemia-reperfusion injury (IRI) is an unavoidable event in organ transplantation after an allograft is implanted. IRI can be exacerbated due to cellular injuries that occur during organ preservation. Yet, preserving donor organs in cold preservation solutions prior to transplantation is the current standard of care in donor organ management. The projects in the Comprehensive Transplant Immunobiology Laboratory (CTIL) are focused on understanding the biology of cellular injuries in the context of organ transplantation and developing novel therapies. Additionally, the CTIL is focused on pre-treatment strategies to condition organs, making them less immunogeneic prior to organ transplantation, to minimize the systemic immunosuppression necessary for the lifetime of transplant recipients.
Role of Cellular Immunometabolism in Organ Transplantation
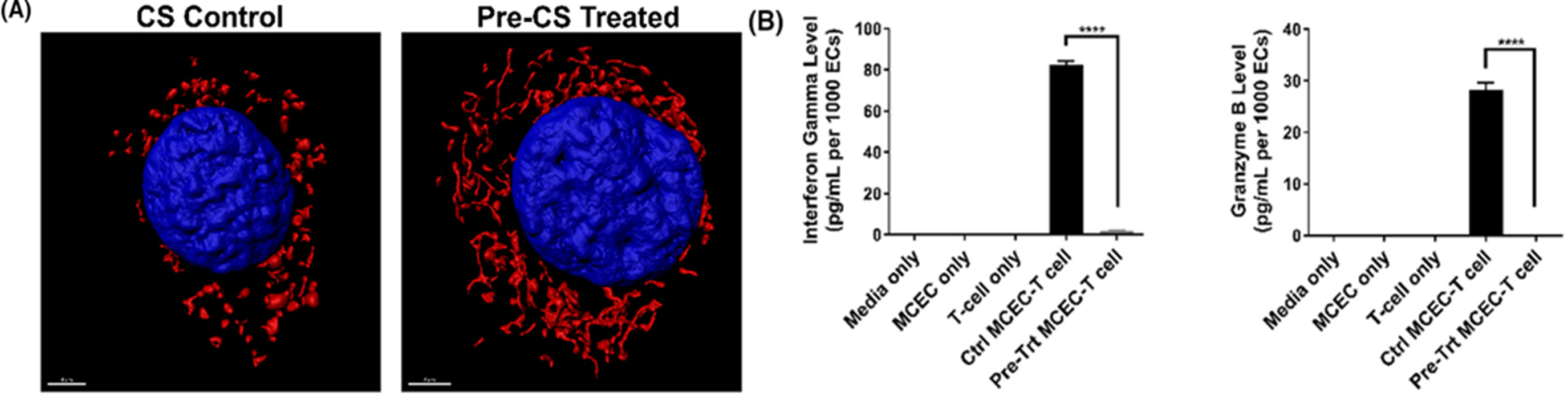
The endothelial cells (EC) lining the blood vessels of an allograft are the first target of early injuries including IRI. We have found that microvascular ECs subjected to IRI cause mitochondrial dysfunction and make them immunogeneic. Specifically, we found that the ECs have depolarized mitochondria and undergo mitochondrial fission causing the ECs to become immunogeneic. Pharmacological modulation of inhibiting mitochondrial fission or promoting mitochondrial fusion significantly reversed this phenotype and the ECs were less immunogeneic. We utilized an in vivo transplantation mouse model to translate our findings wherein donor organs were pre-treated with the pharmacological modulators prior to implantation into recipient mice. This unique pre-treatment strategy significantly improved transplantation outcomes and mitigated early injuries and immunologic responses in the allograft.
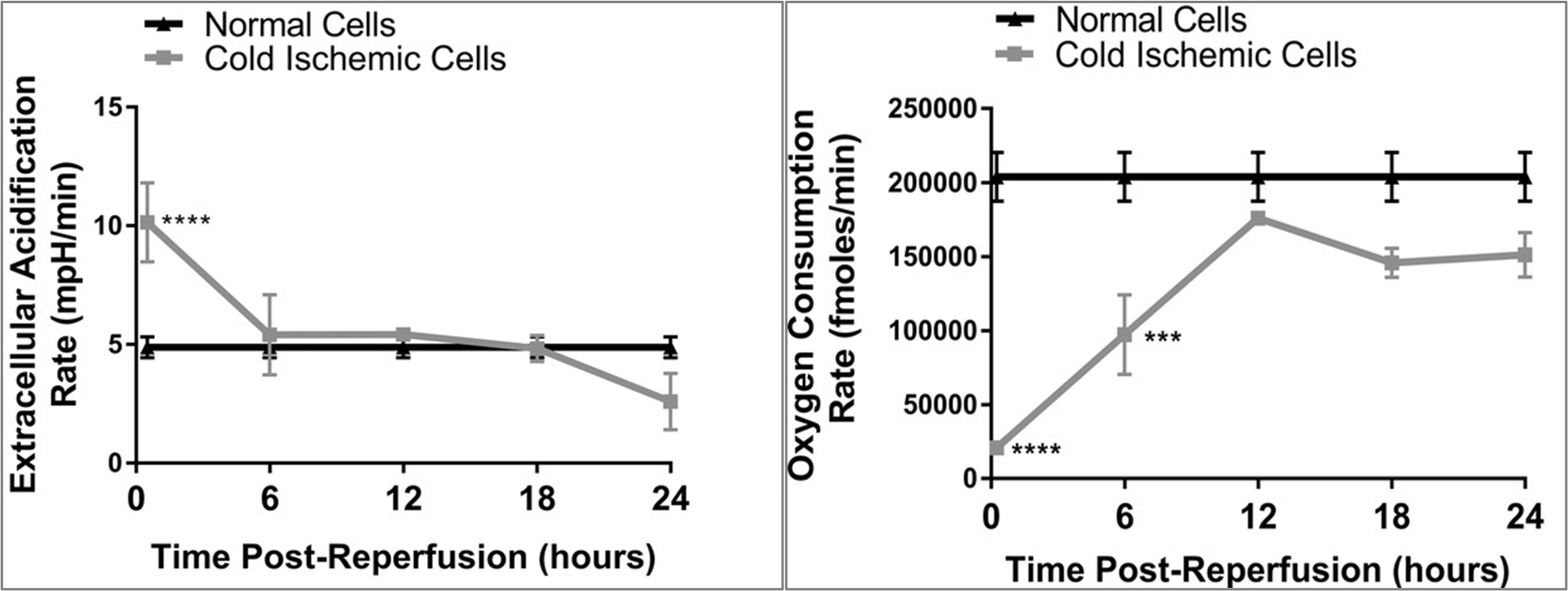
Cold storage and warm reperfusion in ECs induces time-dependent changes in glycolysis (left) and metabolic fitness (right).
Publications: Tran DT et al. Transplantation (2018); Tran DT et al. American Journal of Transplantation (2022)
Bioengineering Nanoparticles for Cell/Tissue-Targeted Delivery
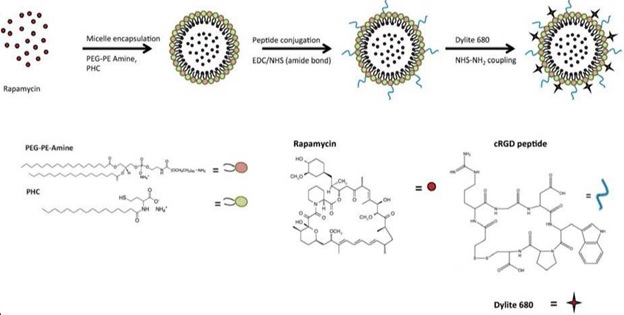
Organ transplant is unique in that the donor organ is isolated for transport and preserved in solutions prior to implantation into the recipient. The period between transport and implantation provides an advantageous opportunity to intervene with therapies that could be applied to the donor organ in the preservation phase without the risk of systemically exposing the therapies to the recipient. To this end, we have developed an EC-targeting nanoparticle encapsulating rapamycin, an immunosuppressive drug, called Targeted Rapamycin Micelle (TRaM). The cyclic arginine-glycine-aspartate (cRGD) moieties on TRaM significantly improved TRaM uptake into ECs and downregulated the production and release of pro-inflammatory cytokines in cold-ischemic injured ECs. Furthermore, pre-treatment of ECs with the NPs was associated with the downregulation of memory T cell responses, a crucial player involved in acute rejection and spared during induction immunosuppression.
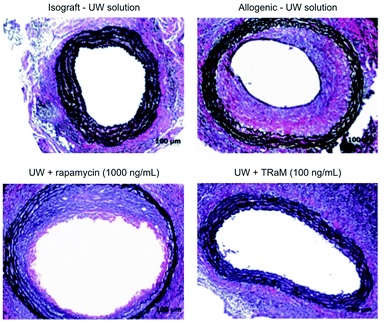
Histological analysis of allografts for vasculopathy – Ex vivo pre-treatment with TRaM nanoparticles in cold University of Wisconsin (UW) organ preservation solution abrogates vasculopathy in allografts.
Publications: Nadig SN et al. RSC Advances (2015); Zhu P et al. RSC Advances (2018)